227 Development of the Endocrine System
Development of the Endocrine System
The endocrine system regulates growth, metabolism, and body homeostasis using hormones that target organs via the bloodstream.
Learning Objectives
Describe the embryological development of the endocrine system
Key Takeaways
Key Points
- The endocrine system is a control system of ductless glands that secretes hormones within specific organs.
- Hormones act as “messengers,” and are carried by the bloodstream to different cells in the body, which interpret these messages and act on them.
- There are two types of hormones secreted in the endocrine system: steroidal (or lipid based) and non-steroidal ( protein -based) hormones.
- The main endocrine glands are the hypothalamus (neuroendocrine gland), pituitary (anterior and posterior lobes), thyroid, parathyroid, adrenal (cortex and medulla), pancreas, and gonads.
Key Terms
- endocrine: Production of internal secretions that are transported around the body by the bloodstream.
- endocrine system: A control system of ductless glands that secrete hormones, which circulate via the bloodstream to affect cells within specific organs.
- hormone: A molecule released by a cell or a gland in one part of the body that sends out messages affecting cells in other parts of the organism.
- homeostasis: The ability of a system or living organism to adjust its internal environment to maintain a stable equilibrium, such as the ability of warm-blooded animals to maintain a constant temperature.
The endocrine system is a control system of ductless glands that secrete hormones within specific organs. Hormones act as messengers, carried by the bloodstream to different cells in the body, which then interpret and act on these messages. Hormones are responsible for the body’s ability to maintain homeostasis and respond to stimuli. Without hormones, you could not grow, maintain a constant temperature, produce offspring, or perform the basic functions essential for life.
The endocrine system provides an electrochemical connection from the hypothalamus of the brain to the organs that control the body’s metabolism, growth and development, and reproduction. The system regulates hormones through negative feedback, except in very specific cases like childbirth. Increases in hormone activity decrease the production of that hormone. The immune system and other factors contribute to maintenance of constant hormone levels. The main endocrine glands are the hypothalamus (neuroendocrine gland), pituitary (anterior and posterior lobes), thyroid, parathyroid, adrenal (cortex and medulla), pancreas, and gonads.
Role of the Thyroid Gland
The thyroid gland is the primary endocrine gland involved in development and one of the largest endocrine glands in the body. It is positioned on the neck just below the larynx and has two lobes, one on either side of the trachea. It is involved in the production of the hormones T3 (triiodothyronine) and T4 ( thyroxine ). These hormones increase the metabolic activity of the body’s cells. A deficiency of iodine in the diet leads to the enlargement of the thyroid gland, known as a simple goiter. The thyroid also produces and releases the hormone calcitonin (thyrocalcitonin), which contributes to the regulation of blood calcium levels. Thyrocalcitonin decreases the concentration of calcium in the blood. Most of the calcium removed from the blood is stored in the bones.

Thyroid system: The thyroid gland controls how quickly the body uses energy, makes proteins, and how sensitive the body is to other hormones.
The thyroid gland is a two-lobed gland that manifests a remarkably powerful active transport mechanism for the uptake of iodide ions from the blood. As blood flows through the gland, iodide is converted to an active form of iodine, which combines with an amino acid called tyrosine. Two molecules of iodinated tyrosine then combine to form thyroxine. The normal thyroid gland may store several weeks supply of thyroxine in this bound form.
Thyroid-Stimulating Hormone and Thyroxine
An enzymatic splitting of the thyroxine from the thyroglobulin occurs when thyroid-stimulating hormone (TSH) produced by the pituitary gland, is released into the blood. TSH stimulates certain major rate-limiting steps in thyroxine secretion. A variety of bodily defects, either dietary, hereditary, or disease-induced, may decrease the amount of thyroxine released into the blood. The most popular of these defects is one that results from dietary iodine deficiency. The thyroid gland enlarges in the continued presence of TSH from the pituitary to form a goiter. This is a futile attempt to synthesize thyroid hormones for iodine levels that are too low. Normally, thyroid hormones act on the pituitary to decrease stimulation of the thyroid via a negative feedback loop. In goiter, the feedback loop is disrupted, leading to continual stimulation of the thyroid and the inevitable protuberance on the neck. The incidence of goiter has been drastically reduced by adding iodine to table salt.
Thyroxine stimulates oxidative metabolism in cells and increases the oxygen consumption and heat production of most body tissues, with the notable exception of the brain. Thyroxine is also necessary for normal growth, likely by promoting the effects of growth hormone on protein synthesis. The absence of thyroxine significantly reduces the ability of growth hormone to stimulate amino acid uptake and RNA synthesis. Thyroxine also plays a crucial role in the closely related area of organ development, particularly that of the central nervous system.
Thyroid Conditions
Hypothyroidism results from an insufficient amount of thyroxine, with symptoms caused by a reduction in the rate of oxidative energy-releasing reactions within the body cells. Hypothyroidism in children, a condition known as cretinism, can result in mental retardation, dwarfism, and permanent sexual immaturity. Sometimes the thyroid gland produces too much thyroxine, a condition known as hyperthyroidism. This condition produces symptoms such as an abnormally high body temperature, profuse sweating, high blood pressure, weight loss, irritability, and muscular pain and weakness. Hyperthyroidism has been treated by partial removal or partial radiation destruction of the gland. More recently, several drugs that inhibit thyroid activity have been discovered, and their use is replacing surgical treatment. Thyroid conditions require lifelong treatment. Both supplementing and suppressing thyroid function can take months or even years to regulate.

The Endocrine System: Major endocrine glands. (Male left, female right. ) 1. Pineal gland 2. Pituitary gland 3. Thyroid gland 4. Thymus 5. Adrenal gland 6. Pancreas 7. Ovary 8. Testis
Aging and the Endocrine System
Three hormone axes are affected by aging: growth hormone/insulin-like growth factor I, cortisol/dehydroepiandrosterone, and testoterone/estradiol.
Learning Objectives
Evaluate the effects of the hormonal axes affected by aging
Key Takeaways
Key Points
- The endocrine system consists of glands and organs that produce and release hormones. Three of the most important hormone axis in the endocrine system affected by aging are growth hormone (GH)/insulin-like growth factor I (IGF-I), cortisol / dehydroepiandrosterone (DHEA), and testosterone / estradiol.
- Somatopause is a term used to describe the change in GH/IGF-I axis, which involves a decrease in production and sensitivity to GH and IGF-I. Typically, GH secretion declines 14% with each decade of life.
- Decline in pituitary GH secretion is associated with loss of skeletal muscle mass, increased adiposity, and other detrimental effects of aging. A decrease in the amount of circulating GH and consequently IGF-I results in weaker bones with reduced density.
- DHEA peaks in the mid-20s and then gradually declines with age (termed adrenopause). Cortisol remains relatively unchanged with aging, causing an imbalance in hormone levels and thus altered immune function.
- Menopause /andropause refers to the decrease in production and circulation of estradiol in females and testosterone in males. In addition to their roles in reproduction and growth, both hormones demonstrate neuroprotective effects and have been theorized to reduce the effects of Alzheimer’s disease.
Key Terms
- insulin-like growth factor I: Also called somatomedin C, a protein that in humans is encoded by the IGF1 gene.
- estradiol: A potent estrogenic hormone ((17)-estra-1,3,5-triene-3,17-diol) produced in the ovaries of female vertebrates; the synthetic compound is used medicinally to treat estrogen deficiency and breast cancer.
- cortisol: Asteroid hormone (also called hydrocortisone) produced by the adrenal cortex that regulates the metabolism of carbohydrates and maintains blood pressure.
- dehydroepiandrosterone: An androgen hormone secreted by the adrenal cortex. A synthetic version is used as a dietary supplement.
- testosterone: Steroid hormone produced primarily in the testes of the male and responsible for the development his of secondary sex characteristics.
The endocrine system consists of glands and organs that produce and release hormones that affect the body in different ways and help control functions including tissue homeostasis, growth and development, reproduction, response to stress, and metabolism.
Three of the most important hormone axes in the endocrine system that are affected by aging include growth hormone (GH)/insulin-like growth factor I (IGF-I), cortisol/dehydroepiandrosterone (DHEA), and testosterone/estradiol.
Growth Hormone (GH) / Insulin-like Growth Factor I (IGF-I) Axis
Somatopause is a term used to describe the change in GH/IGF-I axis, which involves a decrease in production and sensitivity to GH and IGF-I. Typically, GH secretion declines 14% with each decade of life. In the developing human body, GH from the anterior pituitary gland stimulates production and release of IGF-I by the liver, which is then transported in the blood to stimulate growth of muscle and bone.
Decreases in IGF-I signaling, GH deficiency, and GH resistance cause delayed aging and extended lifespan in animal models, in sharp contrast to the effects of GH/IGF-I in humans. Declines in pituitary GH secretion are associated with loss of skeletal muscle mass, increased adiposity, and other detrimental effects of aging in elderly adults. The reason for the opposing actions of GH/IGF-I in different species is not presently understood.
With aging, a decrease in the amount of circulating GH and consequently IGF-I results in weaker bones with a low bone mineral density (BMD). In addition to lower circulating amounts of IGF-I, the responsiveness of bone to this protein has been shown to decrease in animal models. This can be attributed to a decrease in IGF-I signaling pathways with advanced cell age. Binding of IGF-I to its receptors normally initiates signaling cascades involving phosphorylation of extracellular signal related kinase (ERK 1/2) and cyclin-dependent kinase (AKT). These two pathways combine to promote osteoblast proliferation and survival.
Cortisol / Dehydroepiandrosterone (DHEA)
Another hormone axis that changes with aging is the cortisol/DHEA axis. The hypothalmus-pituitary-adrenal pathway plays an integral role in controlling immune function. Two adrenal hormones, DHEA and cortisol, have opposing effects on immune system function, with DHEA generally enhancing immunity and cortisol suppressing it. DHEA is released from the adrenal cortex in response to adrenocorticotrophic hormone (ACTH). DHEA peaks in the mid-20’s and then gradually declines with aging (termed adrenopause), potentially reaching just 5% of its original level. Cortisol remains relatively unchanged with aging, causing an imbalance in hormone levels and thus altered immune function. Glucocorticoids (GCs) such as cortisol also respond to ACTH and are released from the adrenal glands. One specific mechanism in which GCs suppress the immune system is through inhibition of an inhibitor. Nuclear factor kappa B, a transcription factor, inhibits the activation-induced apoptotic response (programmed cell death) that becomes more prevalent with aging. GCs inhibit this transcription factor which in turn decreases inhibition of apoptosis.
Testosterone / Estradiol
Menopause/andropause refers to the decrease in production and circulation of estradiol ( estrogen ) in females and testosterone in males. Testosterone is a steroid hormone secreted by the Leydig cells that can act upon many target organs, resulting in development of secondary sexual characteristics and growth spurt at puberty. Estradiol is the female equivalent of testosterone and is secreted from granulosa cells. It is also a steroid that acts directly on many target organs to develop secondary sexual characteristics and prepare the uterus for potential pregnancy each month.
In addition to their roles in reproduction and growth, both estrogen and testosterone demonstrate neuroprotective effects and have been theorized to reduce the effects of Alzheimer’s disease (AD). AD is characterized by age-related protein deposits in the brain. Specifically, the protein beta-amyloid (Ab) collects in vulnerable brain regions and plays a central role in the progression of AD. In vitro, cells treated with testosterone demonstrated a decrease in Ab release. However, the effects of testosterone are not as potent as that of estrogen. Estrogen acts on the nucleus of the cell by binding with the nuclear endoplasmic reticulum (ER). Once it binds to the ER, a series of activation steps are initiated, resulting in the binding of the estrogen-ER complex to the estrogen responsive element (ERE). This unit mediates expression of neurotrophic factors in the brain, which contributes to neuroprotection. Further, estrogen offers an antioxidant effect at the cellular level in that it can stop oxidation induced by Ab exposure. As a result, the effects of AD are diminished in the presence of both estrogen and testosterone. Thus, a reduction in these hormones with normal aging leaves the brain more vulnerable to AD along with other pathologies. Estrogen levels in particular dramatically plunge with menopause.
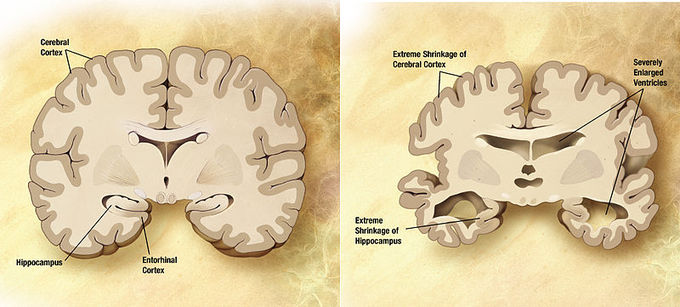
Normal brain vs. brain affected by Alzheimer’s disease: Combination of two brain diagrams in one for comparison. In the left normal brain, in the right brain of a person with Alzheimer’s disease. Estrogen and testosterone demonstrate neuroprotective effects and have been theorized to play a role in reducing the effects of Alzheimer’s disease.
