125 Innate Immunity
Skin and Mucosae (Surface Barriers)
In mammals, the skin and mucosae constitute complex protective barriers that guard against infection and injury.
Learning Objectives
Describe how the skin and mucosae serve as a protective barrier which guards against infection and injury
Key Takeaways
Key Points
- The skin consists of the epidermis, dermis, and basement membrane.
- The epidermis is the outermost layer of skin and forms a protective barrier over the body’s surface. Its layers continually grow outward as older layers shed away.
- The dermis is below the epidermis and is made of connective tissue that cushions the body from stress and strain. It contains mechanoreceptors, blood vessels, and sweat glands.
- The mucosae are linings covered in epithelium and involved in absorption and secretion. They line cavities that are exposed to the external environment and internal organs.
- The barrier immune system is part of the innate immune system, and consists of anything that the skin, mucosae, and chemical secretions of the body do to prevent pathogens from invading.
- The barrier system can fail when the skin breaks or when pathogens invade the mucosal epithelium, so other innate and adaptive immune system functions exist to destroy pathogens in these cases.
Key Terms
- mucous membranes: Specialized epithelium for internal and semi-internal structures that usually secretes mucus and provides some barrier immune system function.
- barrier immune system: A component of the innate immune system that refers to the physical and chemical barriers that prevent pathogens from entering and infecting the body.
The skin is one of the most important body parts because it interfaces with the environment and is the first line of defense from external factors. While it performs a wide range of functions, including sensation, heat regulation, control of evaporation, storage, synthesis, absorption, and water resistance, but its innate immune system functions as the barrier immune system are the most critical.
In humans, the outer covering of the body consists of the skin and mucosae, which together make up the barrier immune system.
Components of the Skin
The skin is made up of several layers that together protect the body, regulate temperature, keep water inside the body, and have sensory function. The skin is the largest organ in the body, and consists of three components that differ greatly in structure and function:
- The epidermis comprises the outermost layers of the skin. It forms a protective barrier over the body’s surface, responsible for keeping water in the body and preventing pathogens from entering. It is made of stratified squamous epithelium tissues, composed of proliferating basal and differentiated suprabasal keratinocytes that form an extracellular matrix that continually divides as the older outer layers of the epidermis shed. The epidermis also helps the skin regulate body temperature through sweat pores that connect to underlying sweat glands in the dermis.
- The basement membrane is a thin sheet of fibers called the basement membrane that separates the dermis and epidermis. It controls the traffic of cells and molecules between the dermis and epidermis and the release of cytokines and growth factors during wound healing.
- The dermis is the layer of skin beneath the epidermis that consists of connective tissue and cushions the body from stress and strain. The dermis provides strength and elasticity to the skin through an extracellular matrix composed of collagen fibrils, microfibrils, and elastic fibers, embedded in proteoglycans. It harbors many mechanoreceptors (sensory nerve endings) that provide the sensations of touch and heat. It also contains the hair follicles, sweat glands, sebaceous glands, apocrine glands, lymphatic vessels, and blood vessels. The blood vessels in the dermis provide nourishment and waste removal for their own cells and for the epidermis.
Mucous Membranes
The mucous membranes (or mucosae; singular mucosa) are linings of mostly endodermal origin, covered in various types of epithelium, that are involved in absorption and secretion. They line cavities that are exposed to the external environment and internal organs. They attached to skin at the nostrils, mouth, lips, eyelids, and genital area, but are also located within the body cavities, such as in the stomach, anus, trachea, and ears.
Most mucous membranes secrete a sticky, thick fluid called mucus, which facilitates several barrier immune system functions and provides a moist environment for internal and semi-internal structures. The mucosae are highly specialized in each organ to deal with different conditions. The most variation is seen in the epithelium. In the esophagus and oropharynx, the epithelium is stratified, squamous and non-keratinising, to protect these areas from harsh or acidic foods. In the stomach it is columnar and organized into gastric pits and glands to secrete acids and pepsin. The small intestine epithelium is specialized for absorption, organized into simple columnar epithelium on protruding villi with narrow crypts that have a high surface area. The mucosal epithelium in the nasopharynx is psuedostratified and ciliated, which helps accumulate and remove mucus.
The Barrier Immune System
Together, the skin and mucosae form the the barrier immune system, technically considered a component of the innate immune system. These structures form physical barriers to infection that prevent pathogens from entering the body through a variety of methods. While the skin simply prevents pathogen entry, more specialized structures like the mucociliary escalator in the trachea trap pathogens in mucus secretions and use cilia to push them out of the trachea to prevent entry into the lungs.
The barrier system also includes chemical barriers that prevent pathogen entry. Notable examples include stomach acidity which kills most microbes, antimicrobial peptides on mucosal epithelial tissue, and even the flow of urine that flushes pathogens out of the urethra.
The barrier system is the first line of defense against pathogen invasion, though it is not perfect. The skin can be broken through cuts or abrasions that expose the bloodstream to the environment. Not every pathogen is caught nor inhibited in mucus, and some may infect the mucosal epithelium directly. Smoking and alcohol abuse damage the muciliary escalator and make it easier for pathogens to invade the lungs. Fortunately, other mechanisms of the innate and adaptive immune systems defend the body when the barrier system fails.

Dermis: A diagrammatic view of a skin section.
Phagocytes
Phagocytes are the white blood cells that protect the body by ingesting harmful foreign particles and help initiate an immune response.
Learning Objectives
Describe the types of phagocytes and their roles in initiating an immune response
Key Takeaways
Key Points
- Many white blood cells and other cells in the body use phagocytosis to engulf and kill cells.
- Phagocytosis occurs over several steps, which include binding to an opsonized pathogen with a receptor and killing it using an oxidative burst.
- Monocytes are phagocytes that can differentiate into macrophages and dendritic cells based on conditions within the body.
- Macrophages are the cleanup crew for the innate immune system. They remove debris, pathogens, and dead neutrophils after an inflammatory response.
- Neutrophils are
polymorphonuclear (PMN) granulocytes that are the first responders to an inflammatory response. They kill pathogens through phagocytosis and degranulation, but die as a result. - Mast cells are circulation PMN granulocytes that trigger an immune response by releasing an inflammatory mediator when they detect an antigen with their toll-like receptors.
Key Terms
- oxidative burst: A chemical reaction that occurs in phagocytes in which an engulfed pathogen is destroyed by exposure to oxidative stress from reactive oxygen species.
- PMN granulocyte: A type of phagocyte that contains PMN granules, most notably neutrophils and mast cells, but also basophiles and eosinophils.
Any cell that undergoes phagocytosis, a process in which pathogens and other foreign particles and debris are engulfed by a cell to be destroyed, is considered a phagocyte. Most phagocytes are types of white blood cells that use phagocytosis to perform basic innate immune system function within the body.
The Mechanism of Phagocytosis
Phagocytosis is the process by which a phagocyte engulfs a pathogen or debris. It can occur in almost any tissue, most often in the bloodstream and interstitial space but also the alveoli of the lungs and the parenchyma of most other major organs in the body. Typical phagocytosis occurs over the course of a few steps:
- A receptor on the phagocyte’s cell membrane binds to a foreign particle, such as a pathogenic microbe or a toxin. The Fc receptor is typically the receptor of use, which binds to antibodies that have opsonized (marked) a pathogen or toxin.
- The cytoplasm surrounds and engulfs the bound pathogen through endocytosis.
- The engulfed pathogen is kept in a vacuole called a phagosome, which then binds to the lysosomes inside the cell.
- A series of chemical reactions called an oxidative burst occurs, which uses reactive oxygen species and NADPH oxidase to damage and kill the pathogen through oxidative stress. Oxidative stress can kill a cell through DNA, cell membrane, or mitochondrial damage.
- The remains of the pathogen are expelled by exocytosis.
These are the general mechanisms used by phagocytosis to engulf and kill pathogens, but some variations can occur. For instance, other receptors may be used to engulf pathogens, and other non-oxidative methods (such as lysozyme) exist to kill the phagocytized pathogen.
Types of Phagocytes
There are many classes of phagocytes within the body, each with different specialized functions involving phagocytosis. Most phagocytes are derived from stem cells in the bone marrow. The main types of phagocytes are monocytes, macrophages, neutrophils, tissue dendritic cells, and mast cells. Other cells, such as epithelial cells and fibroblasts, may also engage in phagocytosis, but lack receptors to detect opsonized pathogens and are not primarily immune system cells.
Monocytes
Monocytes develop in the bone marrow and reach maturity in the blood. Mature monocytes have large, smooth, lobed nuclei and an abundant cytoplasm that contains granules, but are not technically considered granulocytes. Monocytes ingest foreign or dangerous substances and present antigens to other cells of the immune system. Monocytes form two groups: a circulating group and a marginal group that remains in other tissues (approximately 70% are in the marginal group). Most monocytes leave the blood stream after 20–40 hours to travel to tissues and organs; during this process, they differentiate into macrophages or dendritic cells depending on the signals they receive.
Macrophages
Mature macrophages are derived from monocytes, granulocyte stem cells, or the cell division of pre-existing macrophages. Macrophages do not have granules, but contain many lysosomes. They are found throughout the body in almost all tissues and organs, but are rarely found in the bloodstream. Macrophages cause inflammation through the production of interleukin-1, interleukin-6, and TNF-alpha. Macrophages are activated in a number of ways, including by T cells, cytokines such as IFN-gamma, or pathogen-derived compounds such as LPS toxins from bacteria. During inflammation, they enter about 72 hours after the initial response to clean up debris and dead neutrophils.
Dendritic Cells

Dendritic cell: Dendritic cell characterized by membranous projections that resemble spines.
Dendritic cells are specialized antigen-presenting cells that have long outgrowths called dendrites, which help to engulf microbes and other invaders. They express MHC class II molecules, which makes them the ideal antigen-presenting cell. Dendritic cells are present in the tissues that are in contact with the external environment, mainly the skin, the inner lining of the nose, the lungs, the stomach, and the intestines.
Once activated, they mature and migrate to the lymphoid tissues, where they present antigens to T and B cells to initiate the adaptive immune response. This involves deriving T and B cells that are specific towards a single antigen from naive lymphocytes.
Neutrophils
Neutrophils are a type of PMN granulocyte normally found in the bloodstream. They are the most abundant type of phagocyte and the first responder during inflammation. Once they have received the appropriate chemokine signals, neutrophils leave the bloodstream and reach the site of an infection through adhering to the vascular endothelium to squeeze into the tissues. There, they rapidly engulf invaders coated with antibodies, damaged cells, or cellular debris. They also degranulate to release perforin, granzyme, proteases, and other chemicals to cause cytotoxic damage to pathogens (and occasionally normal bodily tissues as well). Neutrophils die after phagocytosis, becoming pus that is later cleaned up by macrophages.

Extravasion of Neutrophils: Neutrophils move through the blood to the site of infection by rolling onto the vascular endothelium and adhering to it to slip through small gaps into the tissues during an inflammatory response.
Mast Cells
Mast cells are PMN granulocytes with toll-like receptors that tend to trigger inflammatory responses. Mast cells express MHC class II molecules and can participate in antigen presentation; however, the mast cell’s role in antigen presentation is not well-understood. Mast cells can consume, kill, and process their antigens. In addition to these functions, mast cells produce cytokines kept in their granules, such as histamine, that induce an inflammatory response when a pathogen is detected. Because of this function, allergic inflammatory responses occur when a mast cell is sensitized to an antigen that it normally wouldn’t react to.

Leukocyte Differentiation: Phagocytes derive from stem cells in the bone marrow. Monocytes differentiate into dendritic cells and macrophages, while mast cells and neutrophils are in a separate group of PMN granuolcytes as well.
Natural Killer Cells
Natural killer (NK) cells are cytotoxic lymphocytes critical for the innate immune system.
Learning Objectives
Describe the role of natural killer cells in the innate immune system
Key Takeaways
Key Points
- NK cells differentiate from lymphocyte progenitor cells and are a critical part of the innate immune system.
- NK cells recognize abnormal or infected cells with activating receptors and inhibitory receptors.
- All normal cells in the body express MHC I to signal that those cells are part of the body.
- Inhibitory receptors recognize MHC class I alleles, which inhibits the NK killing response and explains why NK cells will kill cells possessing few or no MHC class I molecules.
- Activating receptors recognize antigens, antibodies, or other opsonins on a cell’s surface and activate a killing response.
- NK cells are cytotoxic; small granules in their cytoplasm contain proteins such as perforin and proteases known as granzymes that trigger either apoptosis or cell lysis in an abnormal cell.
- NK cells may release two potent immune system cytokines, IFN-gamma and TNF-alpha, when certain receptors are activated.
Key Terms
- Major Histocompatability Complex I: A molecule expressed on cells to signal to immune system cells that they are normal cells of that organism’s body. Abbreviated as MHC I.
- apoptosis: A response in which a cell undergoes programmed cell death and its DNA and other components are destroyed completely. It is a mechanism to stop viral infections and cancer development and is a result of cellular stress.
Natural killer (NK) cells are cytotoxic lymphocytes critical to the innate immune system. The role of NK cells is similar to that of cytotoxic T cells in the adaptive immune response. NK cells provide rapid responses to virus-infected cells and respond to tumor formation by destroying abnormal and infected cells. NK cells use wo cytolytic granule-mediated apoptosis to destroy abnormal and infected cells.
Natural Killer Overview
Typically, immune cells detect major histocompatibility complex (MHC) presented on cell surfaces, triggering cytokine release and lysis or apoptosis in cells that do not express MHC I or express much less of it than normal cells. Unlike phagocytes, NK cells do not need their targets to be opsonized (marked) by antibodies before they can act, allowing for a much faster immune reaction. However, opsonins do speed up the process.
These cells named “natural killers” because they were thought to work without cytokine or chemokine activation. However, later research proved that cytokines play a role in guiding NK cells to stressed cells that may need to be destroyed.
NK cells are large granular lymphocytes derived from the common lymphoid progenitor cells (lymphoblasts), which also generate B and T lymphocytes. NK cells differentiate and mature in the bone marrow, lymph nodes, spleen, tonsils and thymus, where they then enter into the bloodstream.
MHC I Recognition
In order for NK cells to defend the body against viruses and pathogens, they require mechanisms to determine whether a cell is infected. The exact mechanisms remain the subject of current investigation, but recognition of an “altered self” state is thought to be involved. To control their cytotoxic activity, NK cells possess two types of surface receptors: activating receptors and inhibitory receptors. Most of these receptors are also present in certain T cells. These receptors recognize major histocompatability complex I (MHC I), a molecule expressed on every cell to signal that the cell belongs to the body.
When the NK cell recognizes MHC I on a cell using an inhibitory receptor, its killing response is inhibited. When the NK cell does not recognize MHC I on the cell with an inhibitory receptor, or detects an antigen with an activating receptor, the killing response is activated. Virus-infected cells and foreign pathogens such as bacteria and fungi will not express the MHC I specific to the host organism, which will fail to inhibit the NK cell’s killing responses. If both types of receptors are being stimulated, the receptor that experiences a higher degree of relative stimulation will determine the NK cell behavior. Some tumor cells may still express MHC I in low amounts, so they may evade NK cell destruction based on the balance of activating and inhibiting stimuli.

Complementary Activities of Cytotoxic T-cells and NK cells: Schematic diagram indicating the complementary activities of cytotoxic T-cells and NK cells. T-cells are activated by recognizing antigens, while NK cells are activated by not recognizing MHC I.
Mechanisms of Cytotoxicity
The granules of NK cells contain proteins such as perforin and proteases known as granzymes. Upon binding to a cell slated for killing, perforin forms pores in the cell membrane of the target cell, creating an aqueous channel through which the granzymes and associated molecules can enter, inducing either apoptosis or osmotic cell lysis (a form of cell necrosis ). Defensins, an antimicrobial secreted by NK cells, directly kills bacteria by disrupting its cell walls.
Apoptosis is a form of “programmed cell death” in which the cell is stimulated by the cytotoxic mechanisms to destroy itself. Unlike with lysis, apoptosis does not degrade DNA, and cells are destroyed cleanly and completely on their own. Cellular lysis causes necrosis of that cell, in which the DNA and cell components degrade into debris that must be phagocytized by macrophages. This distinction has many important implications. Virus-infected cells destroyed by cell lysis release their replicated virus particles into the body, which infects other cells. In apoptosis, these virus particles are destroyed. However, cancer cells often develop genetic mechanisms to prevent apoptosis signals from occurring, so cell lysis is generally more effective.
Cells that are osponized with antibodies are easier for NK cells to detect and destroy. Antibodies that bind to antigens can be recognized by FcϒRIII (CD16) receptors (a type of activating receptor), resulting in NK activation, release of cytolytic granules, and consequent cell apoptosis.
Cytokines in NK Cell Activity
Cytokines play a role in NK cell activation. Many cells release cytokines as a result of cellular stress when infected with a virus. Cytokines involved in NK activation include IL-12, IL-15, IL-18, IL-2, and CCL5. NK cells are activated in response to interferons or macrophage-derived cytokines. They serve to contain viral infections while the adaptive immune response generates antigen-specific cytotoxic T cells that can clear the infection.
NK cells also secrete their own cytokines of their own to help facilitate immune responses, generally upon NK cell activation. NK cells work to control viral infections by secreting IFNγ (interferon gamma) and TNFα (tumor necrosis factor alpha). IFNγ activates macrophages for phagocytosis and lysis while TNFα acts to promote direct NK tumor cell killing. It is also a potent inflammatory mediator that causes long-lasting inflammatory responses and fever in response to more severe infections. Patients deficient in NK cells prove to be more susceptible to most infections than people with normal levels of NK cells, due to a loss of innate immune system function and efficiency.
Inflammation
Inflammation is part of the biological response of vascular tissues to harmful stimuli.
Learning Objectives
Describe the biological mechanisms of inflammation and its role in innate immunity
Key Takeaways
Key Points
- Acute inflammation occurs due to infection, injury, or irritation, and is an essential part of the healing process to remove pathogens and start the wound-healing process.
- During inflammation, vasodilation occurs, the endothelium becomes more permeable as exudate leaks into the tissues, and neutrophils migrate to the site of inflammation.
- Acute inflammation is initiated by cells already present in all tissues, such as mast cells that recognize pathogen-associated molecular patterns (PAMPs) with toll-like receptors, but other cells like natural killer (NK) cells can trigger inflammation as well.
- Acute inflammation is characterized by pain, redness, immobility (loss of function), swelling, and heat.
- Neutrophils migrate to inflamed tissues by rolling onto the endothelium with selectins, adhering to it with integrins, and sliding through its gaps with PECAM-1. Then they follow chemokines to the tissues to find pathogens to destroy.
- Repeated bouts of acute inflammation lead to chronic inflammation, a process of constant healing and inflammation-induced damage as the initial problem is never truly healed.
- Allergic reactions are the result of an inappropriate immune response that triggers inflammation.
Key Terms
- extravasion:
- exudate: Protein-rich edema caused by proteins flowing into the tissues during inflammation due to increased vascular permeability and oncotic pressure.
- inflammatory mediator: Any chemical released from cells that stimulates the vasodilation and increased permeability that occur during acute inflammation.
Inflammation is part of the complex biological response of vascular tissues to harmful stimuli, such as pathogens, injury or trauma, and irritants. Inflammation is a protective attempt by the organism to remove injurious stimuli and initiate the healing process. Inflammation is not a synonym for infection, even in cases where inflammation is caused by infection. Rather, it refers to the response of the body to try and fight the infection. While inflammation is an important mechanism of innate immunity, it can harm the body in cases of allergy, autoimmunity, and infections in tissues with poor regenerative capacity such as the heart.
Functions and Components of Inflammatory Response
The main function of inflammation is to trigger an immune response in an area of the body that needs it to fight off pathogens that may cause an infection or to help heal an injury. The main symptoms of acute inflammation are swelling, redness, pain, loss of function, and heat. Three components to the basic acute inflammatory response occur every time: vasodilation, increased vascular permeability, and migration of leukocytes to the affected tissues.
Vasodilation
An inflammatory response can be caused by any of numerous inflammatory mediators released from innate immune system cells. The most common short term mediators are histamine and seratonin from mast cells, but bradykinin, complement proteins, some interleukins, prostaglandins, and TNF-alpha may also trigger inflammation from other types of cells. Circulating mast cells contain toll-like receptors, which can detect pathogen-associated molecular patterns (PAMPS) on the surface of pathogens and release an inflammatory mediator such as histamine in response. Alternatively, mast cells may release inflammatory mediators due to signals from damaged cells (which will release clotting factors) during trauma or injury.
After an inflammatory mediator is released in the bloodstream, a period of transient vasoconstriction, lasting only a few seconds, occurs. Then blood vessels expand to undergo vasodilation from the stimulus of the vasoactive inflammatory mediator, which increases blood flow to the area. This causes slowing and stasis of red blood cells, which can be involved in the clotting response needed to stop bleeding in the case of injury. Vasodilation is the reason for the redness, heat, and pain associated with inflammation.
Increased Vascular Permeability
The next step of acute inflammation is an increase in vascular permeability due to inflammatory mediator activity, which causes the blood vessels to become more permeable. Normally only water and small compounds can exit the bloodstream into the tissues, but during inflammation, large proteins in the bloodstream, such as serum albumins, can leak out and into the tissues. Water follows these proteins due to the force of oncotic pressure that the proteins exert. This is called exudate, a form of edema. As exudate accumulates within the tissues, they become swollen. The exudate may carry antimicrobial proteins and antibodies into the tissues, and stimulates lymphatic drainage.
Leukocyte Migration to the Tissues
The next step of the acute inflammatory response is chemotaxis migration of neutrophils to the affected area. Neutrophils are recruited to the site of inflammation by various cytokines. Other inflammatory mediators, such as TNF-alpha and IL-1, increase the expression of adhesion molecules on vascular endothelial cells. The neutrophils loosely attach to the endothelial cells through use of selectins, a process called rolling. Then integrins firmly attach to the adhesion molecules on the endothelial cells, which is called adhesion. Together, rolling and adhesion are referred to as margination, the accumulation of leukocytes on the endothelium.
The next step is for neutrophils to squeeze through the gaps in the endothelium into the tissues through binding with PECAM-1 expressed on the endothelium, a process called extravasion. Then the neutrophils follow a chemotactic gradient to the site of infection of injury in the tissues, where they will degranulate and phagocytize pathogens. Later, macrophages enter the tissues through a similar process to clean up dead neutrophils and cellular debris.
Outcomes of Acute Inflammation

Inflammation: Toes inflamed by chilblains
When acute inflammation ends (typically by release of anti-inflammatory mediators such as IL-10 or an end to the release of inflammatory mediators) resolution will occur if the problem is alleviated. Resolution involves physiological responses that are part of the healing process, such as wound healing. If the problem is not resolved, acute inflammation could occur again. Repeated bouts of acute inflammation, known as chronic inflammation, leads to a progressive shift in the type of cells present at the site of inflammation and is characterized by simultaneous destruction and healing of the tissue from the inflammatory process. In particular, fibrosis (scarring) and tissue necrosis are common outcomes of chronic inflammation.
Antimicrobial Proteins
Antimicrobial peptides are an evolutionarily-conserved component of the innate immune response found among all classes of life.
Learning Objectives
Describe the role of antimicrobial peptides in the innate immune system
Key Takeaways
Key Points
- Antimicrobial peptides include a net positive charge and hydrophilic and hydrophobic ends, which allow them to adhere to the lipid membranes of bacterial cell membranes.
- The positive charge makes antimicrobial peptides selective, so they only adhere to negatively-charged bacterial cell membranes instead of host cell membranes.
- The modes of action by which antimicrobial peptides kill bacteria are varied and include disrupting membranes, interfering with metabolism, and targeting cytoplasmic components.
- Many bacteria have developed antimicrobial resistance, in which a component of their cell membranes or enzyme secretions is altered to prevent the peptides from binding to the bacteria, or by inhibiting the peptides directly.
Key Terms
- antimicrobial resistance: Any mechanism that enables bacteria to evade or inhibit antimicrobial action.
- peptide: A class of organic compounds consisting of various numbers of amino acids in which the amine of one is reacted with the carboxylic acid of the next to form anamide bond.
- amphipathic: A molecule with both hydrophobic and hydrophilic groups that allow it to adhere to lipid structures more easily.
Antimicrobial peptides (also called host defense peptides) are an evolutionarily-conserved component of the innate immune response found among all known species. These peptides are found in many of the mucus membranes across the human body and are therefore considered to be part of the barrier immune system. The function of these peptides is to kill microbial pathogens and prevent them from entering the body.
Structure
Antimicrobial peptides are a unique and diverse group of molecules. As peptides, they consist of chains of amino acids that determine their composition and structure. These peptides have a stronger positive than negative charge, which is an important component of their selectivity. They also include hydrophobic and hydrophilic groups that enable them to latch onto other molecules (often lipids) through intermolecular forces, such as the lipid bilayer that forms cell membranes.
The secondary structures of these molecules follow four themes, including i) α-helical, ii) β-stranded due to the presence of two or more disulfide bonds, iii) β-hairpin or loop due to the presence of a single disulfide bond and/or cyclization of the peptide chain, and iv) extended. Many of these peptides are unstructured and inactive in free solution, and fold into their final configuration upon reaching mucus membranes. The amphipathicity (hydrophilic and hydrophobic ends) and positive charge of peptides are their defining structural features.
Antimicrobial Action
The modes of action by which antimicrobial peptides kill bacteria are varied and include disrupting cell membranes, interfering with metabolism, and damaging organelles. The initial contact between the peptide and target organism is electrostatic due to the force of negative and positive ionic charge. The amino acid composition, amphipathicity, cationic charge, and size allow them to attach to and insert into membrane bilayers to form pores by barrel-stave, carpet, or toroidal-pore mechanisms.
The peptides are selective and thus more likely to adhere to bacterial cell membranes than to cell membranes of the host cells. The peptides have a greater positive charge than negative charge, while bacterial cell membranes have a greater negative charge than host cell membranes. This causes the peptide to bind to bacterial membranes instead of host cell membranes.
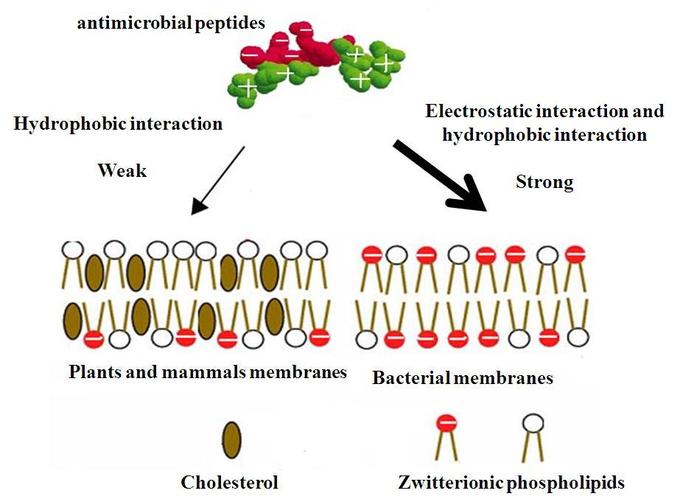
Mechanism of Selectivity of Antimicrobial Peptides: Cell membranes of bacteria are different from the cell membranes of plants and animals and are preferentially targeted by the antimicrobial proteins.

Modes of Action by Antimicrobial Peptides: Antimicrobial peptides multiple various modes of action.
Other antimicrobial mechanisms include intracellular binding models. These involve inhibition of cell wall synthesis, alteration of the cytoplasmic membrane, activation of autolysin, inhibition of DNA, RNA, and protein synthesis, and inhibition of certain bacterial enzymes. However, in many cases, the exact killing mechanism is unknown. In general, the antimicrobial activity of these peptides is determined by measuring the minimal inhibitory concentration (MIC), the lowest concentration of drug that inhibits bacterial growth and an indicator of the antimicrobial strength of that peptide.
Antimicrobial Resistance
Despite the efficiency of antimicrobial peptides to inhibit and kill bacteria, they are still able to get inside the body and cause infections. Bacteria can develop resistance to antimicrobial peptides (as well as separate resistances to antibiotics and other antimicriobials). Bacteria like staphylococcus aureas, which forms the highly resistant MRSA strain, can reduce the negativity of the charge of its cell membrane by bringing amino acids from the cytoplasm into its cell membrane so antimicrobial peptides won’t bind to it. Other forms of antimicrobial resistance include producing enzymes that inhibit the antimicrobial peptides, altering the hydrophobic forces on the cell membrane, and capturing antimicrobial peptides in vesicles on the cell membrane to remove them from the bacterium.
Additionally, commensal bacteria have developed antimicrobial resistance to peptides, but they are normal flora of the body. Most never act as pathogens, though some may be opportunistic pathogens or only act as pathogens in people with certain genetic characteristics.
Fever
Fever is an elevation of body temperature above the regulatory set point, mediated through the release of prostaglandin E2.
Learning Objectives
Describe how fever, a common symptom of medical conditions, is induced by endogenous and exogenous pyrogens
Key Takeaways
Key Points
- Temperature is ultimately regulated in the hypothalamus. A trigger of the fever, called a pyrogen, causes a release of prostaglandin E2 (PGE2). PGE2 then acts on the hypothalamus, which raises the temperature set point so that the body temperature increases through heat generation and vasoconstriction.
- Fever may be useful as an innate immune response to fight off infections by killing bacteria and viruses.
- Aspirin is a potent anti-fever drug because it inhibits COX-2 production, which inhibits PGE2 release.
- A pyrogen is a substance that induces fever and can be either internal (endogenous) or external (exogenous) to the body.
- During severe infections, fever can be more harmful than helpful as the body’s cells are injured in addition to the bacterial cells, which can cause more problems for the innate immune system to handle.
Key Terms
- pyrogen: Any substance that produces fever or a rise in body temperature through the arachidonic acid pathway.
- arachidonic acid pathway: The pathway by which the fever regulator prostaglandin E-2 and several inflammatory mediators are produced by pyrogen activity with phospolipids and COX-2, usually in the brain or liver.
Fever (also known as pyrexia) is a physiological process of the innate immune response against many infections and diseases, characterized by an elevation of temperature above the normal range of 36.5–37.5 °C (98–100 °F) due to an increase in the body temperature regulatory set-point. Although the person’s temperature increases, there is often a feeling of cold. Once the new temperature is reached, there is a feeling of warmth. A fever can be caused by many conditions ranging from benign to potentially serious. Fevers are helpful in fighting infections, but can also cause damage in the body.

Performance of the Various Types of Fever: Performance of the various types of fever: a) Fever continues b) Fever continues to abrupt onset and remission c) Fever remittent d) Intermittent fever e) Undulant fever f) Relapsing fever
Fever Pathways
Temperature is ultimately regulated in the hypothalamus. The primary fever mediator in the human body is prostaglandin E2 (PGE2), which acts on the hypothalamus to raise the temperature set point. PGE2 release comes from the arachidonic acid pathway, which also produces inflammatory mediators such as thromboxane and leukotriene.
This pathway is mediated by the enzymes phospholipase A2 (PLA2), cyclooxygenase-2 (COX-2), and prostaglandin E2 synthase. These enzymes ultimately mediate the synthesis and release of PGE2. Therefore, COX-2 inhibitors such as aspirin are commonly used to reduce fever, although treatments designed to inhibit pyrogens are also effective.
The hypothalamus is the thermostat of the body, in that it alters the temperature set point during temperature feedback and fevers. During a fever, the set point is raised, which causes the body to increase its temperature through both actively generating and retaining heat (vasoconstriction). If these measures are insufficient to make the blood temperature in the brain match the new setting in the hypothalamus, then shivering begins so those muscle movements produce more heat. When the fever stops (when PGE2 release ends), the temperature set point is lowered to normal, and the reverse of these processes (vasodilation, end of shivering, and nonshivering heat production) as well as sweating are used to cool the body to the new, lower setting.
Pyrogens
A pyrogen is a substance that induces fever and can be either internal (endogenous) or external (exogenous) to the body. Pyrogenicity can vary: in extreme examples, bacterial pyrogens known as superantigens can cause rapid and dangerous fevers. Depyrogenation may be achieved through filtration, distillation, chromatography, or inactivation.
Exogenous factors s lipopolysaccharide toxin (from gram negative bacteria) which can activate a number of innate immune activation pathways. These pathways induce the expression of endogenous pyrogens, including a variety of cytokines such as IL1α, IL1β, IL6, TNFα, TNFβ, IFNα, INFβ, and INFγ. For example, if an NK cell detects lipopolysaccharide from a pathogen, it will release TNFα, which will travel through the bloodstream to induce a number of long-lasting inflammatory changes including fever. When TNFα or any of these cytokine factors bind to cells in phospolipids in the brain, the arachidonic acid pathway is activated and PGE2 released to act on the hypothalamus and cause the fever response.
Problems with Fever
Fever is normally a beneficial immune process since increased body temperature can kill off bacteria and viruses and denature bacterial enzymes. But when the body temperature climbs too high, fever is often more harmful than helpful. High fevers also denature the body’s own proteins, which can alter normal cell metabolism, leading to cell injury and death. Persistent high body temperature can also trigger apoptosis. Treatments for severe fevers include antipyrogens and aspirin, which also helps to stop blood clots that may coincide with severe fever.
High fevers (more than 104 degrees Fahrenheit) are a symptom of severe infections. While fevers typically aren’t the direct cause of death in these cases, they do tend to worsen the prognosis. For example, septic shock is a severe bacterial infection in which bacterial toxins stimulate pyrogen and inflammatory mediator activity causes high fever. The fever makes it harder for the body to stop the systemic organ failure that occurs from the compensatory mechanisms in septic shock. Organs fail as blood is pulled away from them to fight the infection (compensatory mechanisms), the damage caused by the fever results in even more compensatory mechanism activity. While septic shock is one of the worst possible examples of fever, it illustrates an important concept in pathophysiology: that normal immune functions are as easily able to hurt us as help us.
