8 Transformations of Matter
Working with Chemical Equations
Chemical Reactions
Terminology
| Reaction Equation: |
The notation used to illustrate a chemical reaction. [latex]2 H_{2} + O_{2} \rightarrow 2H_{2}O[/latex] |
| Reactants: |
Materials consumed in a chemical reaction. [latex]\underline{2 H_{2} + O_{2}} \rightarrow 2H_{2}O[/latex] |
| Products: |
Materials produced in a chemical reaction [latex]2 H_{2} + O_{2} \rightarrow \underline{2H_{2}O}[/latex] |
| States of Matter Notation: | The state that each of the products and reactants are in during the reaction; (s)-solid, (l)-liquid, (g)-gas, (aq)-aqueous. |
We Know: |
Law of Conservation of Mass: |
|
Elements combine in different ways to form substances. All samples of a pure substance contain the same proportion of elements by mass. |
In a chemical reaction atoms are rearranged through the making and breaking chemical bonds, atoms are not altered or destroyed. In a chemical reaction the mass of the products equals the mass of the reactants. |
Law of Conservation of Mass: In a chemical reaction mass is neither created or destroyed.
Balancing Chemical Reactions
We Know: |
Balancing Chemical Equations: |
|
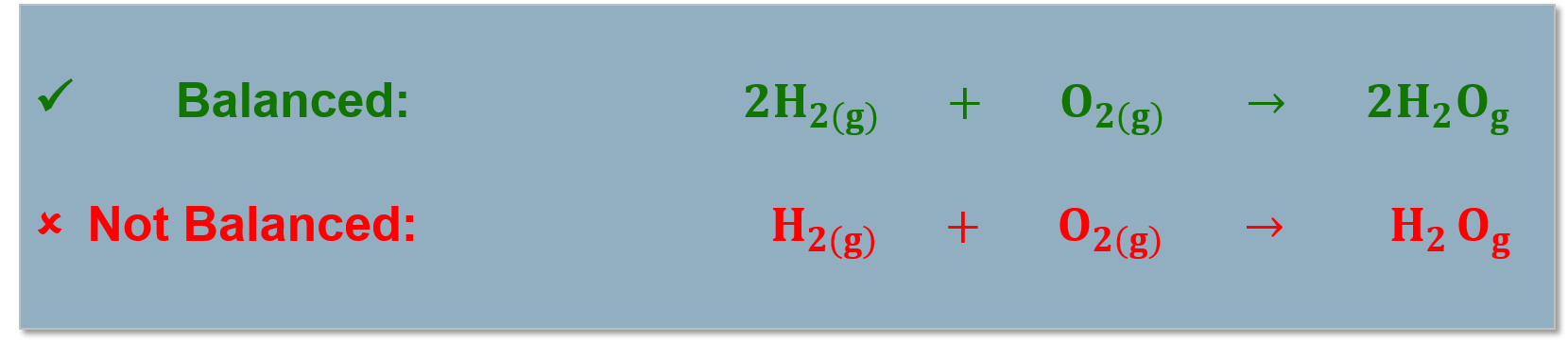
All atoms in the reactants must be accounted for in the products! (Conservation of mass). Chemical reactions are written as reaction equations. |
Reaction equations must be balanced to account for all the products and reactants in the chemical reaction. |
Balancing Chemical Reactions Guide:
Step 1:
Write out the skeletal equation.
[latex]Fe_{(s)} + O_{2(g)} \rightarrow 2 Fe_{2}O_{3(s)}[/latex]
Step 2:
Balance atoms that appear in more complex molecules first.
[latex]Fe_{(s)} +[/latex] [latex]3 O_{2(g)}[/latex] [latex]\rightarrow[/latex] [latex]2[/latex] [latex]Fe_{2}[/latex] [latex]O_{3(s)}[/latex]
Balance oxygen first.
Step 3:
Balance Atoms that appear as free elements.
[latex]4Fe_{(s)}[/latex] [latex]+ 3O_{2(g)} \rightarrow[/latex] [latex]2Fe_{2}[/latex] [latex]O_{3(s)}[/latex]
Balance iron.
Step 4:
Check:
Left side: 4 iron, 6 oxygen
Right side: 4 iron, 6 oxygen
Chemical Reactions Calculations:
We Know: |
Stoichiometry: |
|
Molecular formulas describe the relative number of atoms in a compound. Reaction equations use molecular formulas to describe equations. |
A balanced chemical reaction equation can be used to calculate related reaction quantities! ALWAYS balance reaction equations FIRST in all chemical reaction problems. |
Stoichiometry: Using chemical equations to solve chemical reaction problems.
Limiting Reagent and Chemical Reaction Yield
We Know: |
Limiting Reagent: |
| In a chemical reaction reactants will rearrange in various ways to form products.
A balanced chemical reaction equation can be used to relate reactant and product quantities. What if there is more of one reactant than another? |
In a chemical reaction the reactant that has the smallest amount determines the amount of products that will be produced.
Limiting Reagent: The reactant that limits the amount of products that can be formed in a chemical reaction. |
We Know: |
Yield: |
| Products are formed in a chemical reaction. | The amount of products formed is referred to as a the ‘yield’ of the chemical reaction. |
Terminology
| Theoretical Yield: | The amount of products formed in a chemical reaction based on stoichiometric calculations. |
| Actual Yield: | The amount of products actually formed during a chemical reaction. |
| Percent Yield: |
Actual products formed as a percentage of the theoretical yield. [latex]\text{Percent Yield} = \frac{\text{Actual Yield}}{\text{Theoretical Yield}} \times 100[/latex] |
Solutions
Concentration: |
Molarity: |
Concentration Formula: |
| The amount of dissolved solvent in a specific amount of solute. |
Moles of solute per liter of solution. [latex]M=\frac{mol}{L} = mol\times L^{-1}[/latex] |
[latex]M_{1}V_{1} = M_{2}V_{2}[/latex] [latex]M=\text{molarity}[/latex] |
Many of the chemical reactions we are interested in occur in solution. Chemical problems and laboratories use solutions and solution concentrations frequently!
Solution Terminology
| Solution: | A homogenous mixture of two or more substances consisting of ions or molecules. |
| Solute: | Material present in a solution that has a smaller molar amount. |
| Solvent: | Material present in a solution that has the larger molar amount. |
| Solubility: | The maximum amount of solute that can dissolve in a solvent at a given temperature. |
| Aqueous Solution: | A solution where the solvent is water. |
| Insoluble: | A substance that does not readily dissolve in water. |
| Soluble: | A substance that readily dissolves in water to a significant extent. |
Examples
Problem Set
Below are two documents. One is practice problems, the second is the same problems with solutions.
They can be downloaded and changed to suit your needs.
| Problem Set | Problem Set Solutions |
Quiz
Chemical Reaction Types
Solubility of Ionic Compounds
We Know: |
Solids in Solution: |
|
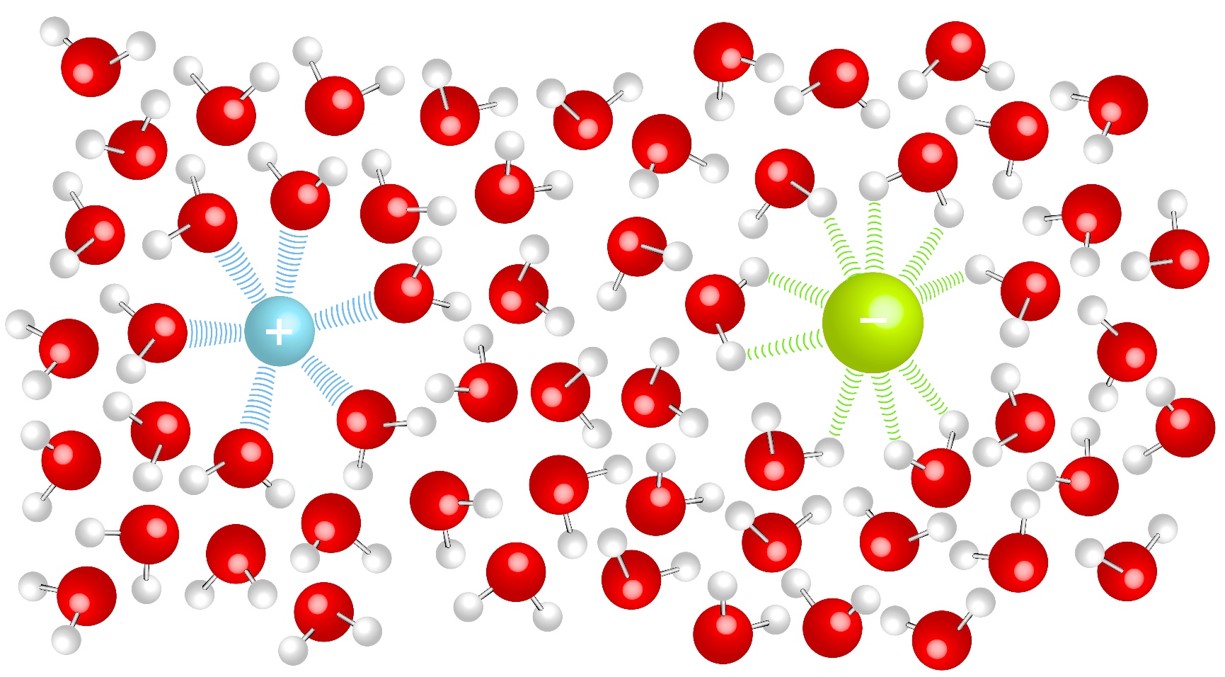
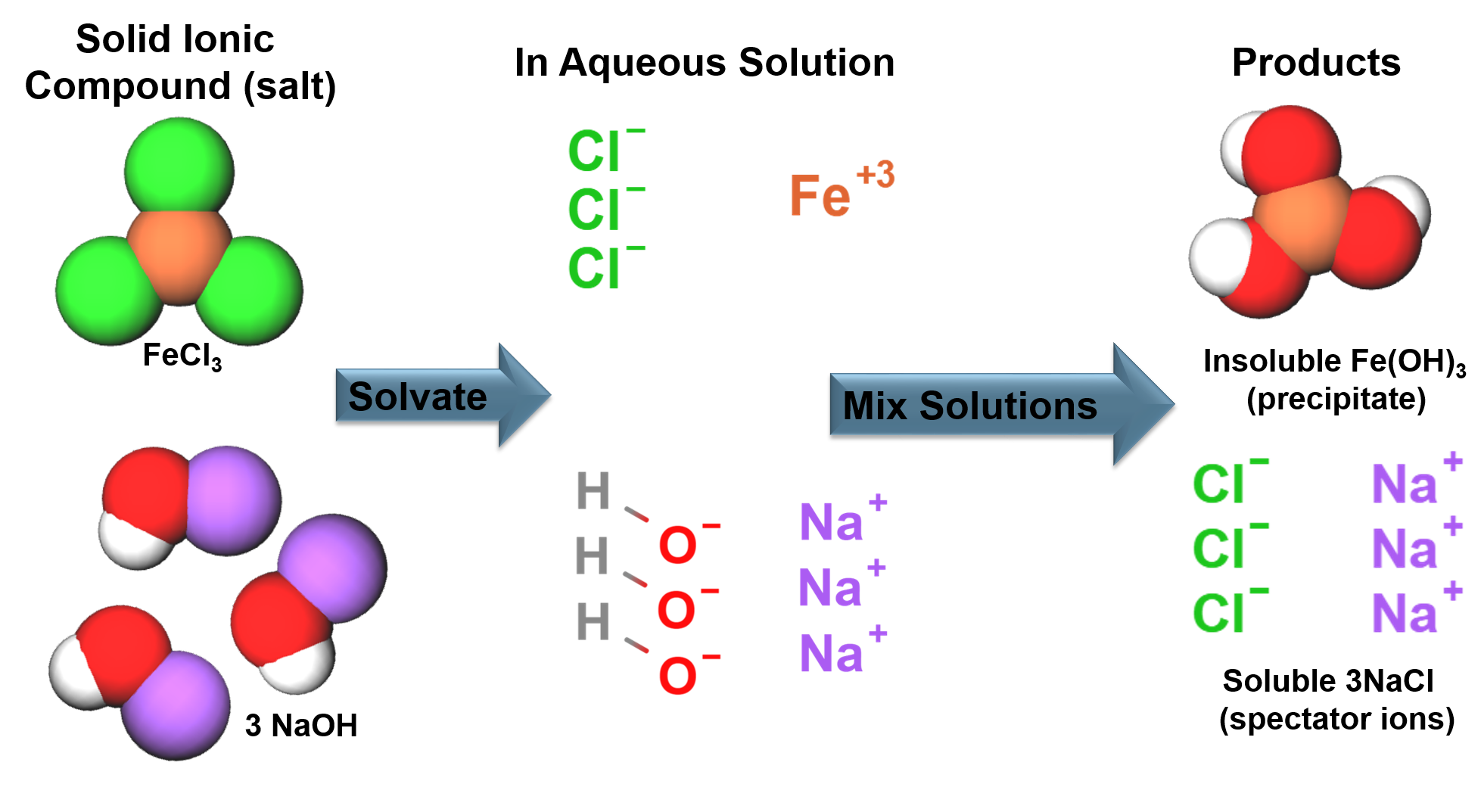
Ionic bonding generally occurs between a metals and non-metals, an electron is transferred from the metal to the nonmetal. The ions formed from the electron transfer are attracted to each other due to electrostatic forces to form a solid. |
Solids in solution will have an attraction to the solvent molecules (solute-solvent interactions). This competes with the forces that hold solids together (solute-solute interactions). The strengths of the interactions will dictate solubility. In aqueous solutions soluble ionic compounds will become free ions surrounded by water molecules. |
Ions in Solution:
| The positive ion will be attracted to the delta negative [latex](\delta -)[/latex] oxygen in the water molecule. | The negative ion will be attracted to the delta positive [latex](\delta +)[/latex] hydrogen in the water molecule. |
There are general rules dictating the solubility of ionic compounds in aqueous solutions.
Products of a Precipitation Reaction
Precipitation of lead (II) iodide:
[latex]2 K I_{(aq)} + Pb(NO_{3})_{2}_{(aq)} \rightarrow 2 KNO_{3}_{(aq)}+PbI_{2}_{(aq)}[/latex]
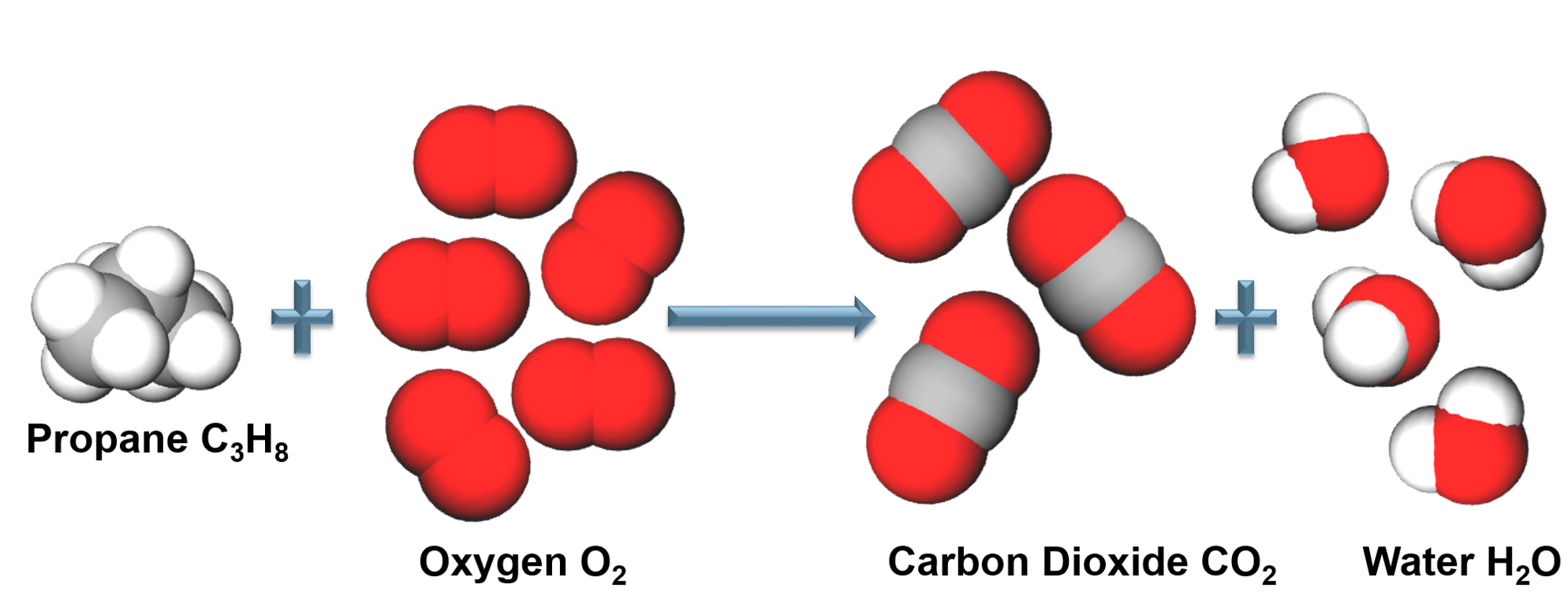
Combustion Reaction
When a reactant combines with oxygen to form one or more compounds containing oxygen.
Acids and Bases
Terminology
| Arrhenius Acid: | A substance that produces [latex]H^{+}[/latex] in an aqueous solution, strong acids completely dissociate in aqueous solution |
| Arrhenius Base: | A substance in an aqueous solution that produces [latex]OH^{-}[/latex], strong bases completely dissociate in aqueous solution. |
| Proton: | The [latex]H^{+}[/latex] ion. |
| Hydroxide: | The [latex]OH^{-}[/latex] ion. |
| Hydronium ion: | The ion formed when a proton associates with water to form [latex]H_{3}O^{+}[/latex]. |
Strong acids and bases will completely dissociate into their respective ions.
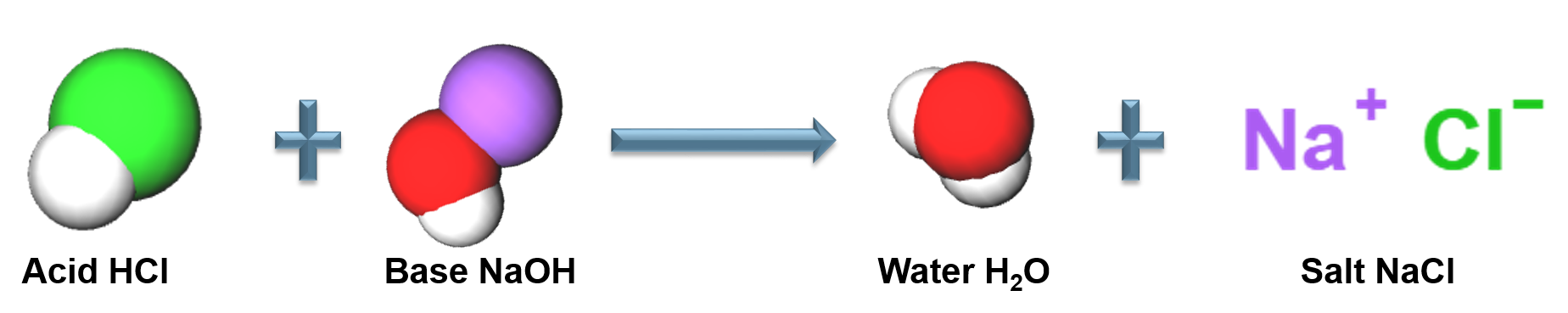
Acid-Base Reactions
A neutralization reaction between an acid and a base
View a list of strong acids and bases.
Arrhenius Acid
A substance that produces [latex]H^{+}[/latex] in an aqueous solution, strong acids completely dissociate in aqueous solution
[latex]HCl_{(s)} + H_{2}O_{(l)} \rightarrow H^{+}_{(aq)} + Cl^{-}_{(aq)}[/latex]
Arrhenius Base
A substance in an aqueous solution that produces [latex]OH^{-}[/latex], strong bases completely dissociate in aqueous solution.
[latex]NaOH_{(aq)} + H_{2}O_{(l)} \rightarrow OH^{-}_{(aq)} + Na^{+}_{(aq)}[/latex]
Hydronium ion:
The ion formed when a proton associates with water to form [latex]H_{3}O^{+}[/latex].
[latex]H^{+}_{(aq)} + H_{2}O_{(l)} \rightarrow H_{3}O^{+}_{(aq)}[/latex]
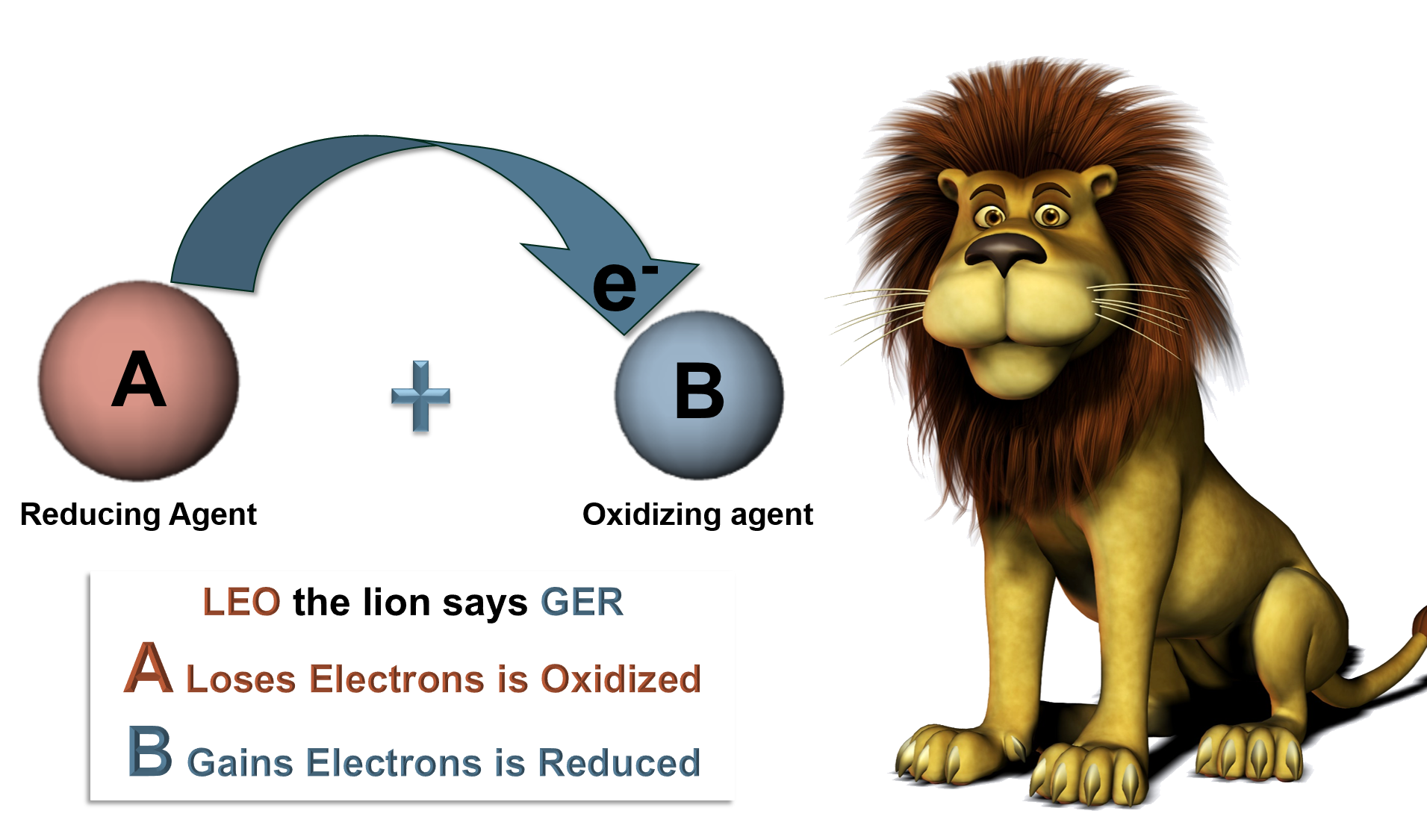
Oxidation Reduction Reactions
Terminology
| Oxidation: | The loss of electrons. |
| Reduction: | The gain of electrons. |
| Oxidant: | Gains electrons, is the oxidizing agent. |
| Reductant: | Loses electrons, is the reducing agent. |
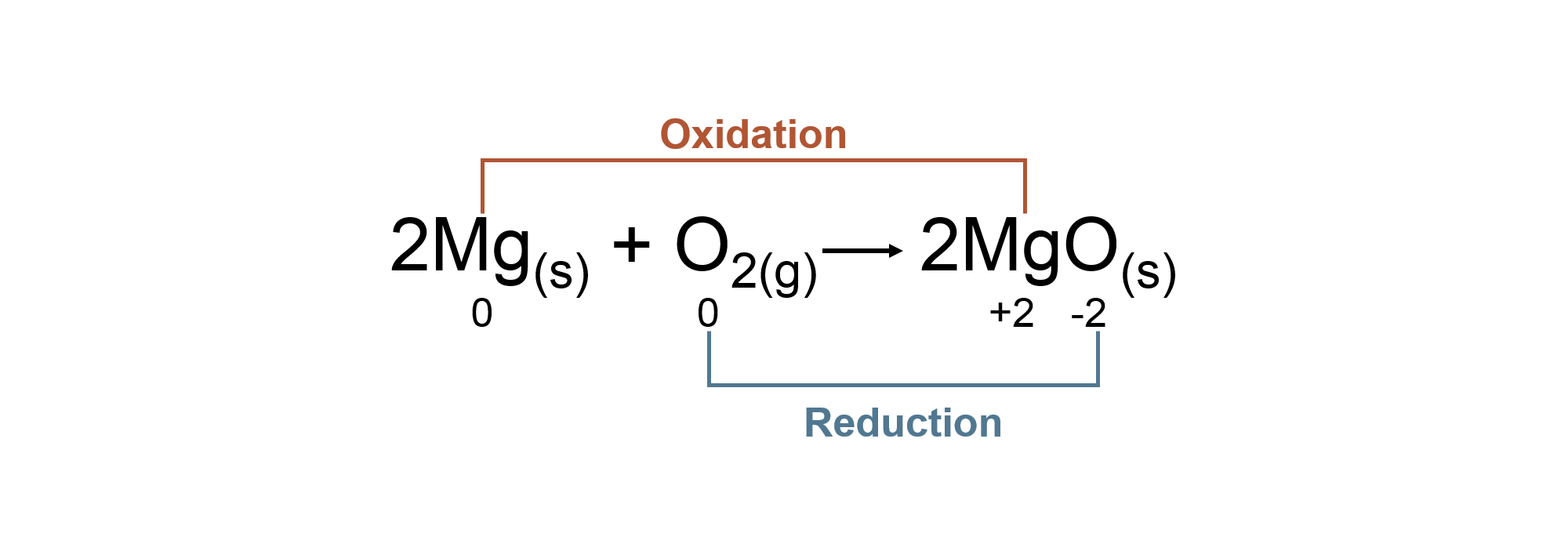
Oxidation States
| Oxidation states of an atom is a system designed by chemists to track electrons during chemical reactions. | An atom is assigned shared electrons based on its electronegativity (ability to attract electrons). | The oxidation state given to an atom is based on the electrons assigned to it. | There are specific rules to follow in assigning oxidation states. |
Oxidation states are a bookkeeping method for tracking electrons in chemical reactions. It does not model the actual placement of an electron in a compound!
Rules:
Rules must be followed in order! Rule 1 takes precedence over Rule 2 etc…
1. |
2. |
3. |
| The oxidation of an atom in an elemental state is zero (0). | Monoatomic ions have an oxidation state is equal to the ion’s charge. |
For compounds, the SUM of the oxidation states of all atoms in:
i. A neutral molecule is zero (0). ii. A polyatomic ion is equal to the sum of the ion’s charge. |
4. |
5. |
6. |
| Metals in compounds have positive oxidation states. i. Group 1 metals have an oxidation state of (+1). ii. Group 2 metals have an oxidation state of (+2). |
Hydrogen generally has a (+1) oxidation state. | In compounds non-metals generally have negative oxidation states: i. Fluorine always has a (-1) oxidation state. ii. The remaining group 17 elements generally have a (-1) oxidation state. iii. Oxygen generally has an oxidation state of (-2). iv. The remaining group 16 elements generally have an oxidation sate of (-2). v. Group 15 elements generally have an oxidation state of (-3). |
For elements not covered in the rules, use rule three to determine the oxidation state once all other oxidations states have been assigned.
Oxidation Reduction Reaction with Oxidation States
Coefficients do not affect the oxidation state of an atom.
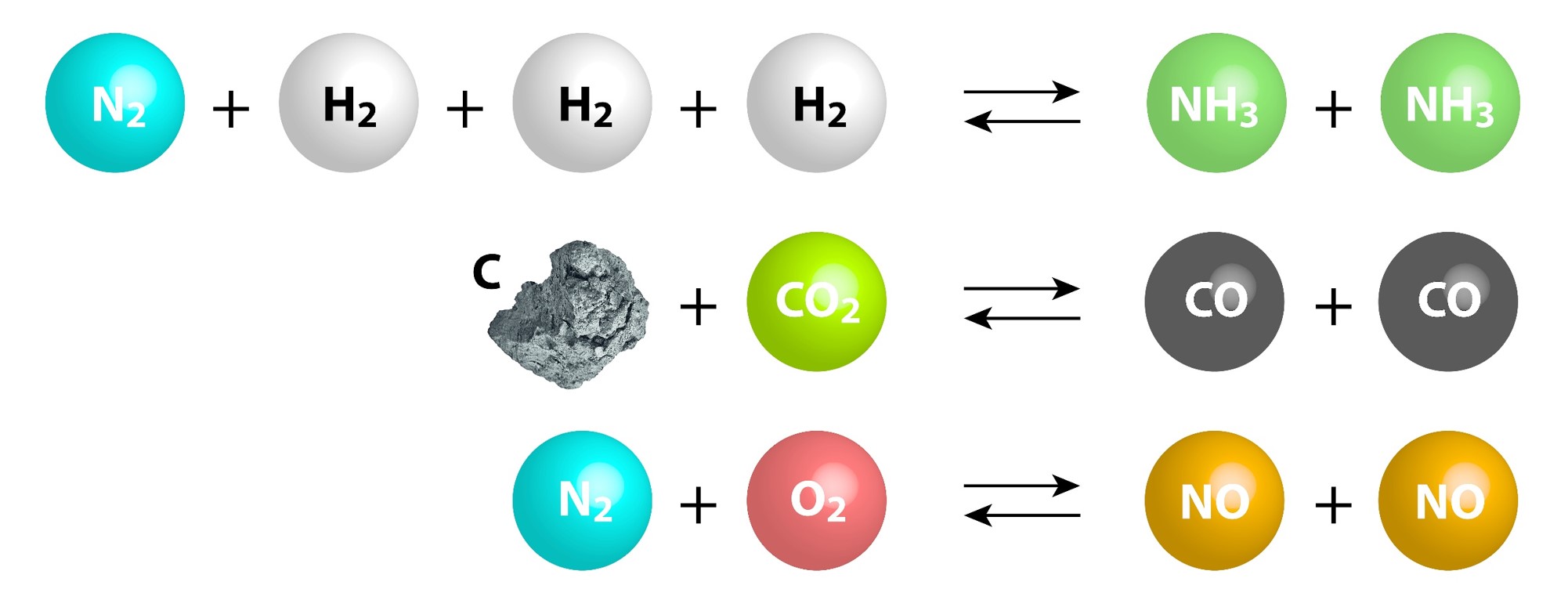
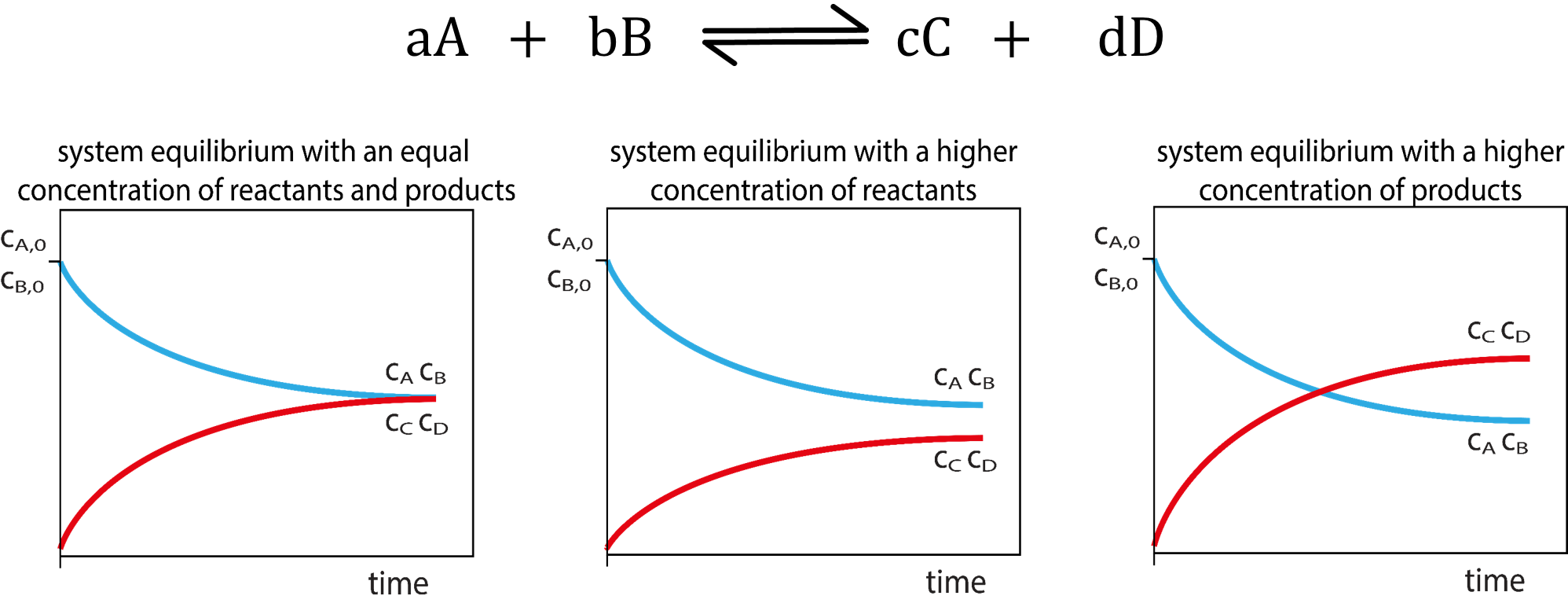
Chemical Equilibrium Concepts
| Certain reactions are reversible and can proceed in the forward and reverse directions. | The number of forward reactions occurring could initially be fast and numerous. | Over time the chances of the reverse reaction occurring increases with the increased amount of products formed. |
| Eventually a point in time will be reached when the forward reactions equal the reverse reactions. | ||
| When a reaction has reached equilibrium it has not stopped, the forward and reverse reactions are continually occurring. | When the number of reactions per unit of time (rate) of forward reactions is equal to the number of reverse reactions per unit of time (rate) the system is said to be in dynamic equilibrium. | When a system has reached dynamic equilibrium the concentrations of the products and reactants do not change. (This does not mean the concentrations are equal to each other!) |
Examples
Problem Set
Below are two documents. One is practice problems, the second is the same problems with solutions.
They can be downloaded and changed to suit your needs.
| Problem Set | Problem Set Solutions |
Quiz
Media Attributions
- reaction © Adobe Stock
- balancing_equation
- solutions © Adobe Stock
- ions_in_solution © Adobe Stock
- Products_of_Precipitation
- combustion_reaction
- acid_base_reaction
- oxidation_reduction © Adobe Stock
- oxidation_states
- chemical_equilibrium_concept © Adobe Stock