9 Summary
Take the Test
Review Module 2 Material
Modern Atomic Theory Review
Cation (+): |
Isotopes: |
Anion (-): |
|
One or more atoms that has lost an electron(s). A main group METAL will LOSE electrons to form a CATION with the same number of electrons as the nearest noble gas. |
Are atoms of the same type that have a different number of neutrons. Isotopes of elements are distinguished by the mass number (A). |
Elements that have unpredictable properties. A main group NONMETAL will GAIN electrons to form an ANION with the same number of electrons as the nearest noble gas. |
Atomic Mass: |
We know: |
|
Is the weighted average of isotopic masses based on the natural abundance of each isotope. The natural abundance is the relative amount of each isotope found in a natural sample of any element. Natural abundance is relatively constant and unique for each element. |
Isotopes are atoms of the same type that have a different number of neutrons. The mass of an atom is dictated by the mass of protons and neutrons because the mass of an electron is negligible in comparison. |
Atomic Mass Calculations:
Atomic mass for an element can be calculated with the following formula:
[latex]\sum_{n} (\text{fraction of isotope } n) \times (\text{mass of isotope } n)[/latex]
In simple terms this formula is telling us that atomic mass can be calculated by:
|
1) Multiplying each isotopic mass of an element by its relative abundance. |
2) Adding the resulting masses of the isotopes together. |
Defining the Mole:
The mole is defined using the C-12 isotope.
One mole = number of atoms in exactly 12 g C-12.
12 g of C-12 = 1 mole of C-12 atoms = exactly [latex]6.02 \times 10^{23}[/latex] C-12 atoms
The mass of one mole of C-12 atoms is 12 g
1 mole of carbon (C) = [latex]6.02 \times 10^{23}[/latex] atoms
Periodic Trends
Metals: |
Non-Metals: |
Metalloids: |
|
Generally good conductors of heat and electricity Tend to lose electrons in a chemical reaction |
Generally poor conductors of heat and electricity Tend to gain electrons in a chemical reaction |
Have properties of both metals and non-metals. |
Main Group: |
Transition Metals: |
Lanthanoids and Actinoids: |
| Elements that have predictable properties | Elements that have unpredictable properties. | Elements that are placed separately for a more compact periodic table. They have similar properties to the elements lanthanum and actinium. |
Groups
|
Each column in the periodic table is called a group and numbered 1-18 |
Each row in the periodic table is called a row and numbered 1-7 |
The elements in a group have similar properties. |
For example: The noble gases in group 18 are all relatively unreactive. |
Atomic Structure
Orbital: |
Spin Quantum Number [latex]m_{s}[/latex] |
| The likely position of an electron in an atom described by a probability distribution map. |
Spin is intrinsic to all electrons, it is quantized, with only two orientations; spin up or spin down. Value = [latex]+\frac{1}{2}[/latex] OR [latex]-\frac{1}{2}[/latex] |
Principal Quantum Number (n) |
Angular Momentum Quantum Number (l) |
Magnetic Quantum Number [latex](m_l)[/latex] |
|
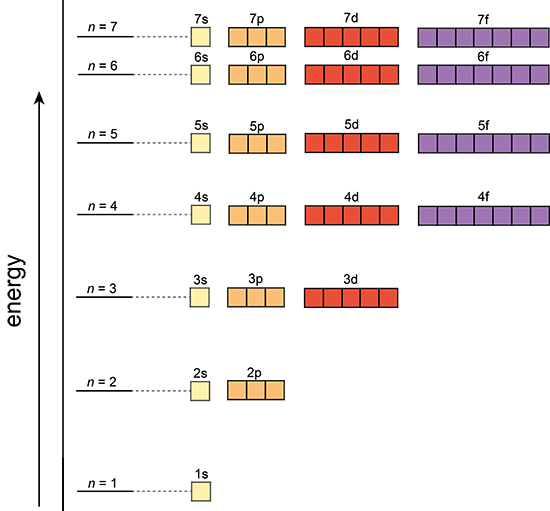
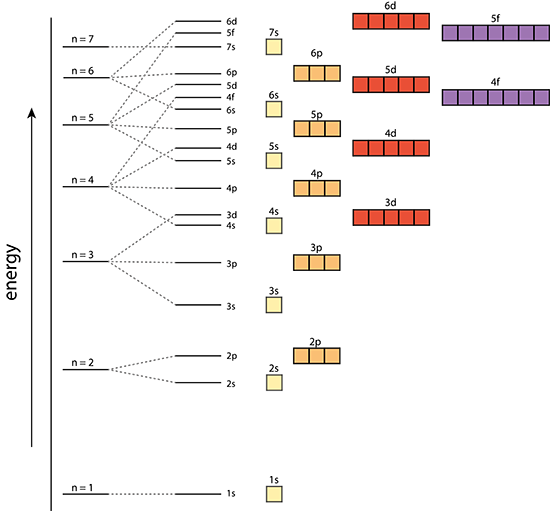
Orbital’s size and energy level of an electron. A whole number 1,2,3,4….. n increases with an electron’s energy and distance from the nucleus. Each level is called a shell. |
The shape of an orbital. 0,1,2,3,…,n-1 Each value for l is a subshell Each number value of l is assigned a letter s,p,d,f,g…. |
Orientation of orbital in 3D space. Value= integer values from –l…l. There are 2(l)+1 values of [latex]m_l[/latex] for each value of l. |
Orbital Shapes
Orbital Shapes = Angular Momentum Number (l)
| Values of (l) angular momentum | |
| Number | Letter |
| 0 | s |
| 1 | p |
| 2 | d |
| 3 | f |
| 4 | g |
Letter values continue according to the alphabet after f, with the exception of J.
s,p,d,f,g,h,i,k,….. Sober Physicists Don’t Find Giraffes Hiding In Kitchens
Pauli Exclusion Principle
No two electrons in an atom can have the same four quantum numbers, however, Any three quantum numbers can be the same.
This implies: One orbital may contain two electrons of opposite spin orientation!
 |
 |
Single electron atom |
Multi-electron atom |
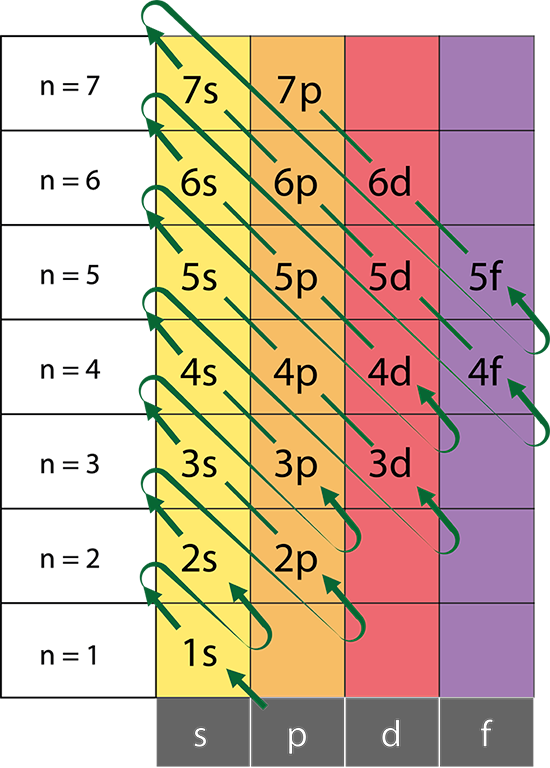
Aufbau Principle
Electrons occupy lower energy orbitals before filling higher energy orbitals.
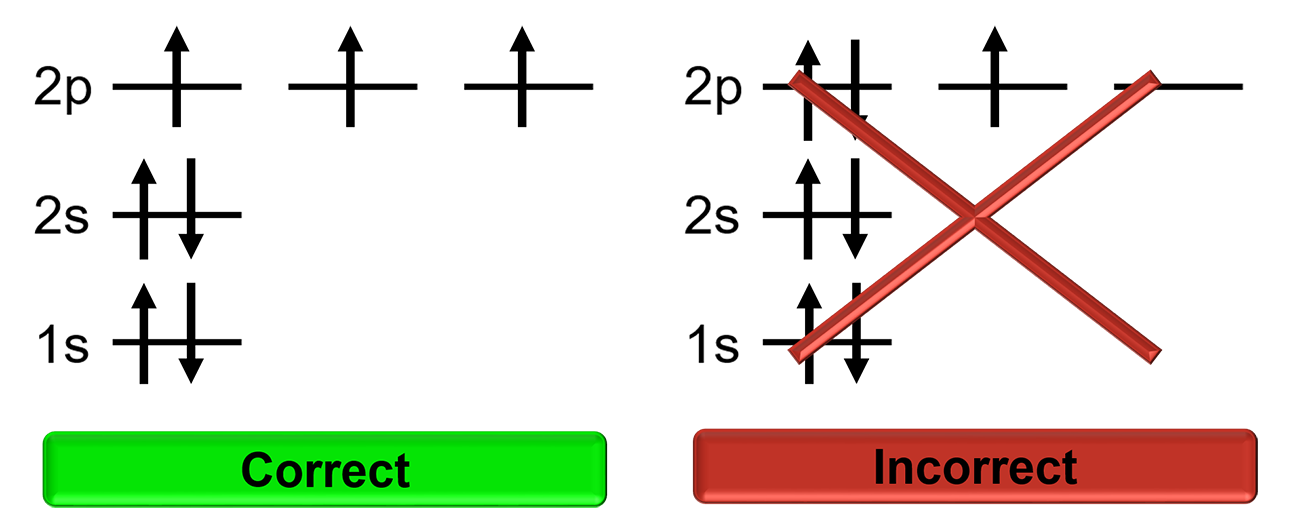
Hund’s Rule
Electrons will singly occupy degenerate orbitals until they are half full before pairing up with other electrons. Electrons in half full orbitals will have the same spin.
Building the World Review
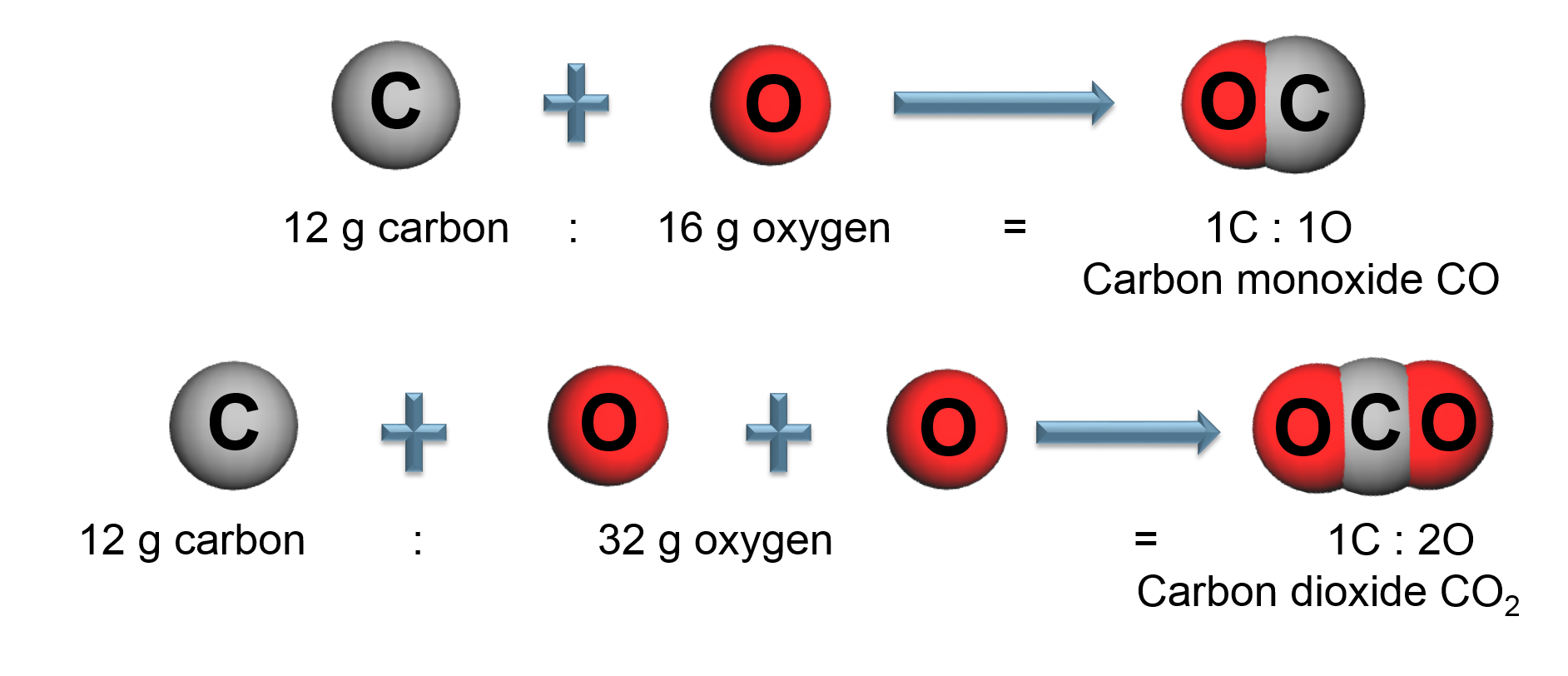
The Law of Multiple Proportions
Elements combine in different ways to form substances whose mass ratios are small whole number multiples of each other.
The Law of Definite Proportions
All samples of a pure chemical substance always contain the same proportion of elements by mass regardless of their origin.
By mass, Carbon dioxide ([latex]CO_{2}[/latex]) is: 73% Oxygen and 27% Carbon
All carbon dioxide is chemically the same in every location.
Pure chemical substances are chemically the same.
Empirical Formula |
Molecular Formula: |
NOTE |
|
The lowest whole integer numbers representing an atomic ratio of a molecule using a chemical description. Empirical Formula of hydrogen peroxide: [latex]HO[/latex] |
A chemical description of the actual complete molecule. Molecular Formula of hydrogen peroxide: [latex]H_{2}O_{2}[/latex] |
Some compounds have empirical and molecular formulas that are the same. Empirical Formula of water: [latex]H_{2}O[/latex] |
Periodic Trends:
The periodic table is organized to help us determine useful information about elements. For example: atomic radius, electronegativity, and ionization energy. Learning periodic trends can help us understand why certain elements have the properties we observe.
Electronegativity
| The ability of a bonded atom to attract electrons | Moving down a column on the periodic table electronegativity decreases | Moving across a row on the periodic table electronegativity increases |
| The difference in electronegativity determines bond type |
Electronegativity differences (ΔEN) for bonded atoms can be calculated by subtracting the least electronegative atom from the atom with the highest electronegativity. |
For hydrochloric acid (HCl): Electronegativity of Cl=3.0 Electronegativity of H=2.1 ∆𝐄𝐍=𝟑.𝟎-𝟐.𝟏=𝟎.𝟗 |
Atomic Radius
| Is the bonding radius determined from averaging measurements of many compounds and molecules | The bond length of a two bonded atoms is determined by adding their bond radii | Moving down a column on the periodic table the principle quantum number (n) of the outermost electrons increases, orbitals are larger, therefore the atomic radii are larger. | Moving across a row the electrons in the outermost shell feel the charge from the nucleus more strongly. Electrons are closer to the nucleus, orbitals are smaller, therefore the radii are smaller |
On the periodic table bond radius increases down a column.
On the periodic table bond radius decreases across a row.
Atomic Radius and Ions
| An atom that has lost an electron (cation) will have a smaller radius than the neutral atom. Fewer electrons = smaller electron cloud = smaller ionic radius. | An atom that has gained an election (anion) will have a larger radius than the neutral atom. More electrons = larger electron cloud = larger ionic radius. |
Cations have a smaller ionic radius than the neutral atom.
Anions have a larger ionic radius than the neutral atom.
Ionization energy
| The amount of energy required to remove one electron from an atom (or ion) in a gaseous state. | Moving down a column on the periodic table the principle quantum number (n) of the outermost electrons increase, orbitals are larger, therefore it takes less energy to remove an electron because they are farther from the nucleus. | Moving across a row the electrons in the outermost shell feel the charge from the nucleus more strongly, orbitals are smaller, therefore it takes more energy to remove an electron because they are closer to the nucleus. |
On the periodic table ionization energy decreases down a column.
On the periodic table ionization energy increases across a row.
Chemical Bonding
Chemical Bonding Terminology
 |
 |
| Two or more bonded atoms. | Two or more bonded atoms of the same type. |
 |
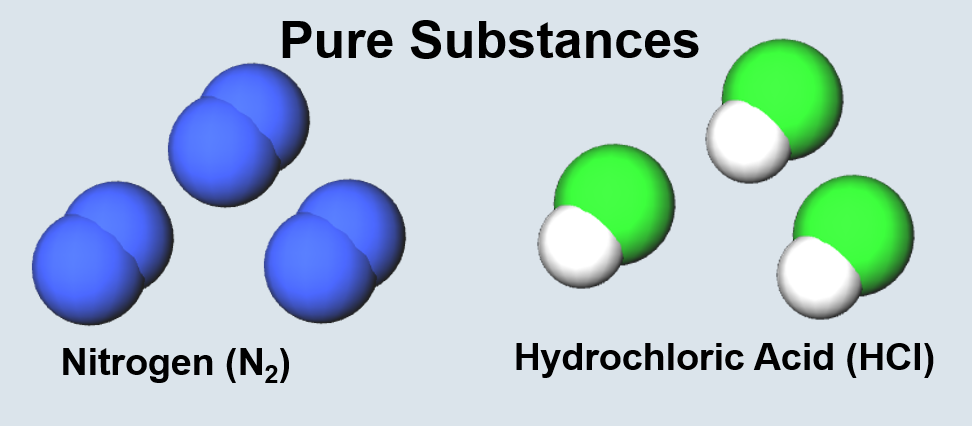 |
| Two or more bonded atoms of different types. | Two or more molecules or atoms of the same type. |
Classes of Chemical Bonding
Electronegativity and Bond Types
The electronegativity difference ([latex]\Delta EN[/latex]) between bonded atoms can be used to identify which type of bond exists in a molecule.
Electronegativity Differences and Bond Type
| ([latex]\Delta EN[/latex]) | Bond Type | Example |
| 0.0-0.4 | Covalent (no charge on atoms) | [latex]O_2[/latex] Oxygen |
| 0.4-2.0 | Polar Covalent (partial charge on atoms) | [latex]CO[/latex] Carbon Monoxide |
| 2.0 + | Ionic (full charge on ions) | [latex]KI[/latex] Potassium Iodide |
Valence Electrons and Bonding
We know: |
Valence Electrons: |
Valence Electrons Continued: |
| Valence electrons are the electrons in the outermost shell of an atom. |
The valence electrons are the electrons involved in chemical bonding between atoms. The number of valence electrons an element has determines the chemical properties of that element. Elements in a column (group) have the same number of valence electrons, this is why elements in a group have similar chemistry. |
The valence electrons of a main group element are located in the outermost shell. The valence electrons of a transition metal are located in the outermost d orbitals, as well as the outermost shell. |
Valence electrons are responsible for the chemical properties of an atom.
Valence Electrons and Lewis Structures
Lewis Structures: |
Lewis Theory: |
Octet: |
| Representing the valence electrons of a main group element using dots surrounding the chemical symbol. |
Chemical bonding is the attainment of a stable electron configuration through the sharing or transfer of electrons. Lewis Theory uses the octet rule to predict bonding. Lewis structures can be used to demonstrate bonding in molecules by showing atoms in a molecule sharing electrons to attain a full octet. |
A full outer shell containing eight electrons. |
The Octet Rule
|
A stable electron configuration can be attained with eight electrons in the outermost shell. Bonding atoms will transfer or share electrons to satisfy the octet rule, each atom will have access to eight electrons in its outermost shell. The octet rule can be used to predict how atoms bond. |
This rule only works for the second period (row) of the periodic table. Elements beyond the second row can access d or f orbitals, elements are larger and have more room for bonding. |
Hyper-coordination: Elements beyond the second row (after Neon) can have expanded octets. The octet rule is a useful tool for predicting bonding in molecules especially in organic chemistry. |
Lewis Structures Terminology
| Formal Charge: |
The charge on an atom in a Lewis structure, assuming all electrons are shared equally. Formal charge is calculated with the following formula: [latex](\text{valence }e^{-}) - (\frac{1}{2}\text{bonding }e^{-}) - (\text{lone electrons}) = \text{Formal Charge}[/latex] Formal charge is used to determine the best Lewis structure for a compound that has more than one choice. The lowest possible charge indicates the most energetically favorable structure. |
| Resonance Structures: |
When more than one Lewis structure is allowed for a compound drawing both structures with a double headed arrow between them is the correct way to indicate resonance. |
Transformations of Matter Review
Chemical Reactions Terminology:
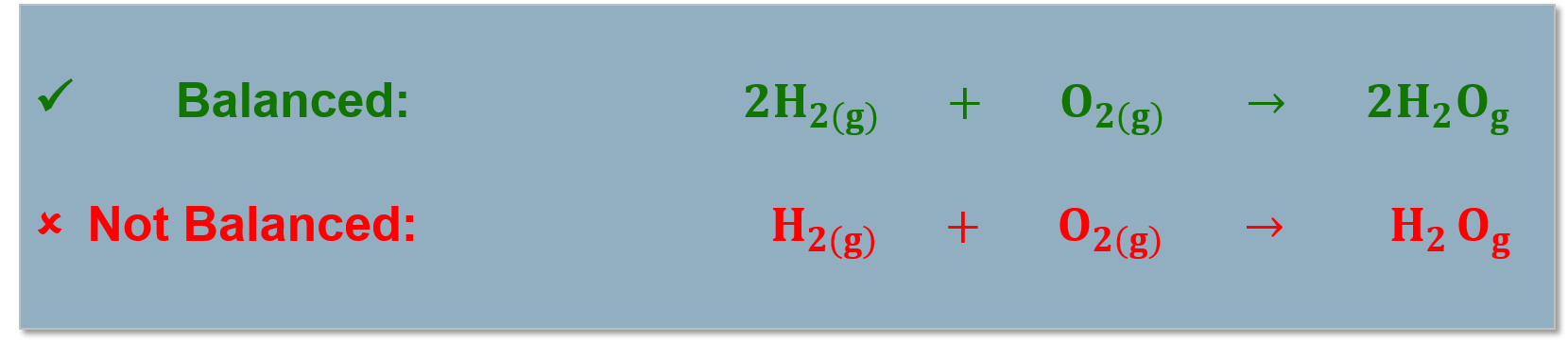
| Reaction Equation: |
The notation used to illustrate a chemical reaction. [latex]2 H_{2} + O_{2} \rightarrow 2H_{2}O[/latex] |
| Reactants: |
Materials consumed in a chemical reaction. [latex]\underline{2 H_{2} + O_{2}} \rightarrow 2H_{2}O[/latex] |
| Products: |
Materials produced in a chemical reaction [latex]2 H_{2} + O_{2} \rightarrow \underline{2H_{2}O}[/latex] |
| States of Matter Notation: | The state that each of the products and reactants are in during the reaction; (s)-solid, (l)-liquid, (g)-gas, (aq)-aqueous. |
Limiting Reagent Terminology:
| Theoretical Yield: | The amount of products formed in a chemical reaction based on stoichiometric calculations. |
| Actual Yield: | The amount of products actually formed during a chemical reaction. |
| Percent Yield: |
Actual products formed as a percentage of the theoretical yield. [latex]\text{Percent Yield} = \frac{\text{Actual Yield}}{\text{Theoretical Yield}} \times 100[/latex] |
Solution Terminology:
| Solution: | A homogenous mixture of two or more substances consisting of ions or molecules. |
| Solute: | Material present in a solution that has a smaller molar amount. |
| Solvent: | Material present in a solution that has the larger molar amount. |
| Solubility: | The maximum amount of solute that can dissolve in a solvent at a given temperature. |
| Aqueous Solution: | A solution where the solvent is water. |
| Insoluble: | A substance that does not readily dissolve in water. |
| Soluble: | A substance that readily dissolves in water to a significant extent. |
Law of Conservation of Mass:
In a chemical reaction atoms are rearranged through the making and breaking chemical bonds, atoms are not altered or destroyed.
In a chemical reaction the mass of the products equals the mass of the reactants.
Law of Conservation of Mass: In a chemical reaction mass is neither created or destroyed.
Balancing Chemical Reactions
We Know: |
Balancing Chemical Equations: |
|
All atoms in the reactants must be accounted for in the products! (Conservation of mass). Chemical reactions are written as reaction equations. |
Reaction equations must be balanced to account for all the products and reactants in the chemical reaction. |
Balancing Chemical Reactions Guide:
Step 1:
Write out the skeletal equation.
[latex]Fe_{(s)} + O_{2(g)} \rightarrow 2 Fe_{2}O_{3(s)}[/latex]
Step 2:
Balance atoms that appear in more complex molecules first.
[latex]Fe_{(s)} +[/latex] [latex]3 O_{2(g)}[/latex] [latex]\rightarrow[/latex] [latex]2[/latex] [latex]Fe_{2}[/latex] [latex]O_{3(s)}[/latex]
Balance oxygen first.
Step 3:
Balance Atoms that appear as free elements.
[latex]4Fe_{(s)}[/latex] [latex]+ 3O_{2(g)} \rightarrow[/latex] [latex]2Fe_{2}[/latex] [latex]O_{3(s)}[/latex]
Balance iron.
Step 4:
Check:
Left side: 4 iron, 6 oxygen
Right side: 4 iron, 6 oxygen
Chemical Reaction Types
Different Chemical Reaction Types
Precipitation of lead (II) iodide:
[latex]2 K I_{(aq)} + Pb(NO_{3})_{2}_{(aq)} \rightarrow 2 KNO_{3}_{(aq)}+PbI_{2}_{(aq)}[/latex]
Combustion Reaction:
When a reactant combines with oxygen to form one or more compounds containing oxygen.
|
Arrhenius Acid |
A substance that produces [latex]H^{+}[/latex] in an aqueous solution, strong acids completely dissociate in aqueous solution [latex]HCl_{(s)} + H_{2}O_{(l)} \rightarrow H^{+}_{(aq)} + Cl^{-}_{(aq)}[/latex] |
|
Arrhenius Base |
A substance in an aqueous solution that produces [latex]OH^{-}[/latex], strong bases completely dissociate in aqueous solution. [latex]NaOH_{(aq)} + H_{2}O_{(l)} \rightarrow OH^{-}_{(aq)} + Na^{+}_{(aq)}[/latex] |
|
Hydronium ion |
The ion formed when a proton associates with water to form [latex]H_{3}O^{+}[/latex]. [latex]H^{+}_{(aq)} + H_{2}O_{(l)} \rightarrow H_{3}O^{+}_{(aq)}[/latex] |
Oxidation State Rules:
Rules must be followed in order! Rule 1 takes precedence over Rule 2 etc…
1. |
2. |
3. |
| The oxidation of an atom in an elemental state is zero (0). | Monoatomic ions have an oxidation state is equal to the ion’s charge. |
For compounds, the SUM of the oxidation states of all atoms in:
i. A neutral molecule is zero (0). ii. A polyatomic ion is equal to the sum of the ion’s charge. |
4. |
5. |
6. |
| Metals in compounds have positive oxidation states. i. Group 1 metals have an oxidation state of (+1). ii. Group 2 metals have an oxidation state of (+2). |
Hydrogen generally has a (+1) oxidation state. | In compounds non-metals generally have negative oxidation states: i. Fluorine always has a (-1) oxidation state. ii. The remaining group 17 elements generally have a (-1) oxidation state. iii. Oxygen generally has an oxidation state of (-2). iv. The remaining group 16 elements generally have an oxidation sate of (-2). v. Group 15 elements generally have an oxidation state of (-3). |
For elements not covered in the rules, use rule three to determine the oxidation state once all other oxidations states have been assigned.
Chemical Equilibrium Concepts
| Certain reactions are reversible and can proceed in the forward and reverse directions. | The number of forward reactions occurring could initially be fast and numerous. | Over time the chances of the reverse reaction occurring increases with the increased amount of products formed. |
| Eventually a point in time will be reached when the forward reactions equal the reverse reactions. | ||
| When a reaction has reached equilibrium it has not stopped, the forward and reverse reactions are continually occurring. | When the number of reactions per unit of time (rate) of forward reactions is equal to the number of reverse reactions per unit of time (rate) the system is said to be in dynamic equilibrium. | When a system has reached dynamic equilibrium the concentrations of the products and reactants do not change. (This does not mean the concentrations are equal to each other!) |
Media Attributions
- neutron © Adobe Stock
- proton © Adobe Stock
- electron © Adobe Stock
- energy levels (2) © Adobe Stock
- energy levels © Adobe Stock
- Aufbau © Adobe Stock
- hunds
- Multiple_Proportions
- molecule
- molecular_elements
- molecular_compounds
- pure_substances
- chemical_bond © Adobe Stock
- covalent_bond
- polar_covalent_bond1
- balancing_equation
- combustion_reaction