Chapter 2 Minerals
Learning Objectives
After reading this chapter, completing the exercises within it, and answering the questions at the end, you should be able to:
- Describe the nature of atoms and their constituents, particularly the behaviour of electrons and the formation of ions.
- Apply your understanding of atoms to explain bonding within minerals.
- Describe mineral lattices and explain how they influence mineral properties.
- Categorize minerals into groups based on their compositions.
- Describe a silica tetrahedron and the ways in which tetrahedra combine to make silicate minerals.
- Differentiate between ferromagnesian and other silicate minerals.
- Explain some of the mechanisms of mineral formation.
- Describe some of the important techniques for identifying minerals.
Minerals are all around us: the graphite in your pencil, the salt on your table, the plaster on your walls, and the trace amounts of gold in your computer. Minerals can be found in a wide variety of consumer products including paper, medicine, processed foods, cosmetics, electronic devices, and many more. And of course, everything made of metal is also derived from minerals.
As defined in Chapter 1, a mineral is a naturally occurring combination of specific elements arranged in a particular repeating three-dimensional structure (Figure 1.4.1).
“Naturally occurring” implies that minerals are not artificially made. Many minerals (e.g., diamond) can be made in laboratories, but if they can also occur naturally, they still qualify as minerals.
“Specific elements” means that most minerals have a specific chemical formula or composition. The mineral pyrite, for example, is FeS2 (two atoms of sulfur for each atom of iron), and any significant departure from that would make it a different mineral. But many minerals can have variable compositions within a specific range. The mineral olivine, for example, can range all the way from Fe2SiO4 to FeMgSiO4 to Mg2SiO4. Intervening compositions are written as (Fe,Mg)2SiO4 meaning that Fe and Mg can be present in any proportion, and that there are two of them for each Si present. This type of substitution is known as solid solution.
Most important of all, a mineral has a specific “repeating three-dimensional structure” or “lattice,” which is the way in which the atoms are arranged. We’ve already seen in Chapter 1 how sodium and chlorine atoms in halite alternate in a regular pattern. That happens to be about the simplest mineral lattice of all; most mineral lattices are much more complicated, as we’ll see.
Some substances that we think must be minerals are not because they lack that repeating 3-dimensional structure of atoms. Volcanic glass is an example, as is pearl or opal. As shown in Figure 2.0.1, opal appears to have a regular structure, but it’s not an atomic structure.
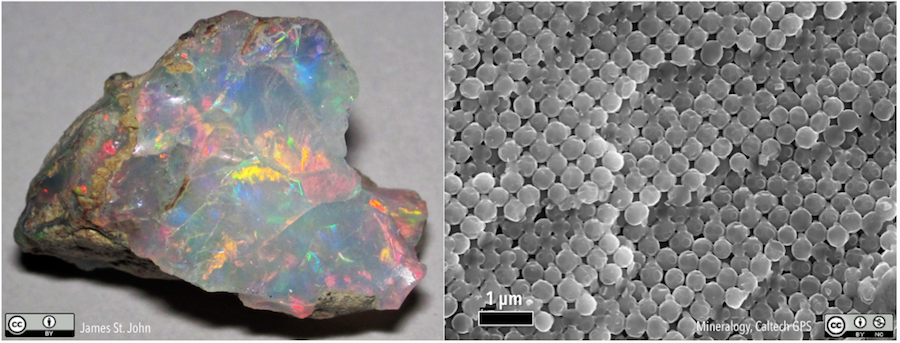
Media Attributions
- Figure 2.0.1 (left): Precious opal. © James St. John. CC BY.
- Figure 2.0.1 (right): Opal beads. © Mineralogy Division, Geological and Planetary Sciences, Caltech. CC BY-NC.
the existence of foliation in a metamorphic rock
