60 10.1 Alfred Wegener: The Father of Plate Tectonics

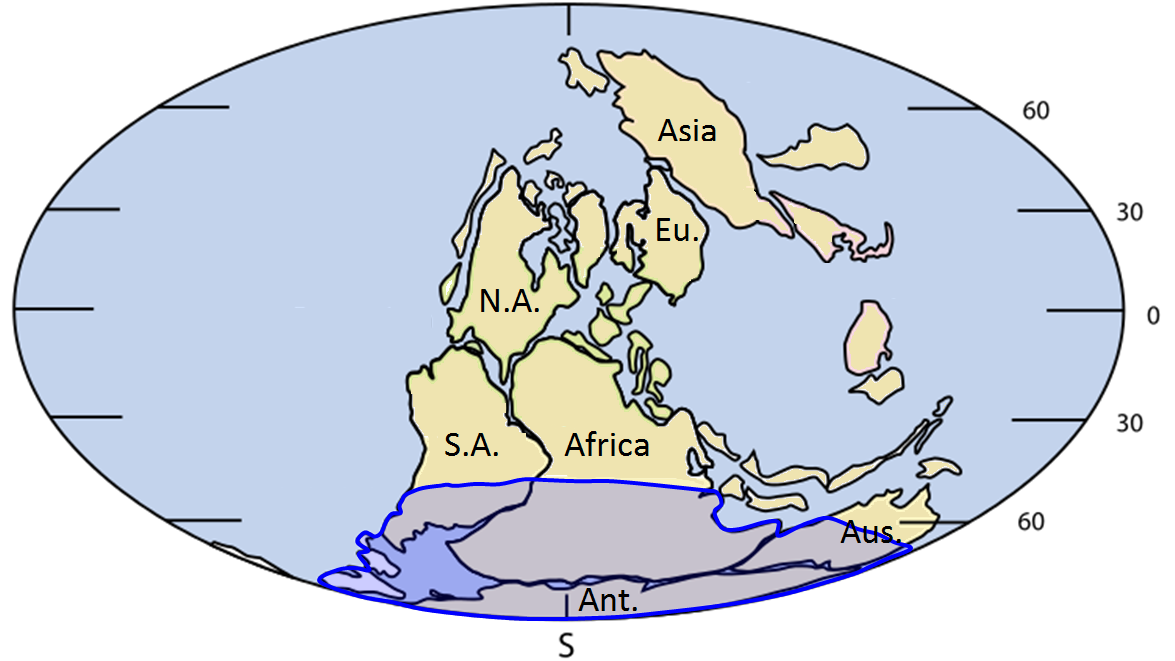
Alfred Wegener (1880-1930) (Figure 10.1.1) earned a PhD in astronomy at the University of Berlin in 1904, but he had always been interested in geophysics and meteorology and spent most of his academic career working in meteorology. In 1911 he happened on a scientific publication that included a description of the existence of matching Permian-aged terrestrial fossils in various parts of South America, Africa, India, Antarctica, and Australia (Figure 10.1.2).
Wegener concluded that this distribution of terrestrial organisms could only exist if these continents were joined together during the Permian, and he coined the term Pangea (“all land”) for the supercontinent that he thought included all of the present-day continents.

Wegener pursued his theory with determination—combing the libraries, consulting with colleagues, and making observations—looking for evidence to support it. He relied heavily on matching geological patterns across oceans, such as sedimentary strata in South America matching those in Africa (Figure 10.1.3), North American coalfields matching those in Europe, and the mountains of Atlantic Canada matching those of northern Britain—both in morphology and rock type.

Wegener referred to the evidence for the Carboniferous and Permian (~300 Ma) Karoo Glaciation in South America, Africa, India, Antarctica, and Australia (Figure 10.1.4). He argued that this could only have happened if these continents were once all connected as a single supercontinent. He also cited evidence (based on his own astronomical observations) that showed that the continents were moving with respect to each other, and determined a separation rate between Greenland and Scandinavia of 11 metres per year, although he admitted that the measurements were not accurate. In fact they weren’t even close—the separation rate is actually about 2.5 centimetres per year!
Wegener first published his ideas in 1912 in a short book called Die Entstehung der Kontinente (The Origin of Continents), and then in 1915 in Die Entstehung der Kontinente und Ozeane (The Origin of Continents and Oceans). He revised this book several times up to 1929. It was translated into French, English, Spanish, and Russian in 1924.
In fact the continental fits were not perfect and the geological matchups were not always consistent, but the most serious problem of all was that Wegener could not conceive of a credible mechanism for moving the continents around. It was understood by this time that the continents were primarily composed of sialic material (SIAL: silicon and aluminum dominated, similar to “felsic”), and that the ocean floors were primarily simatic (SIMA: silicon and magnesium dominated, similar to “mafic”). Wegener proposed that the continents were like icebergs floating on the heavier SIMA crust, but the only forces that he could invoke to propel continents around were poleflucht, the effect of Earth’s rotation pushing objects toward the equator, and the lunar and solar tidal forces, which tend to push objects toward the west. It was quickly shown that these forces were far too weak to move continents, and without any reasonable mechanism to make it work, Wegener’s theory was quickly dismissed by most geologists of the day.
Alfred Wegener died in Greenland in 1930 while carrying out studies related to glaciation and climate. At the time of his death, his ideas were tentatively accepted by only a small minority of geologists, and soundly rejected by most. However, within a few decades that was all to change. For more about his extremely important contributions to Earth science, visit the NASA website to see a collection of articles on Alfred Wegener.
Image Descriptions
Figure 10.1.2 image description: Fossils found across different continents suggest that these continents were once joined as a super-continent. Fossil remains of Cynognathus (a terrestrial reptile) and Mesosaurus (a freshwater reptile) have been found in South America and Africa. Fossil evidence of the Lystrosaurus, a land reptile from the Triassic period, has been found in India, Africa, and Antarctica. Fossils of the fern Glossopteris have been found in Australia, Antarctica, India, Africa, and South America. When you position these continents so they fit together, the areas where these fossils were found line up. [Return to Figure 10.1.2]
Media Attributions
- Figure 10.1.1: “Alfred Wegener ca.1924-30.” Public domain.
- Figure 10.1.2: “Snider-Pellegrini Wegener fossil map” by Osvaldocangaspadilla. Public domain.
- Figure 10.1.3: © Steven Earle. CC BY. Based on “Angola -Brazil sub-sea geology” by Cobalt International Energy can be found at U.S. Energy Information Administration: Country Analysis Brief: Angola (May 2016) [PDF].
- Figure 10.1.4: “Karoo Glaciation” © GeoPotinga. Adapted by Steven Earle. CC BY-SA.
the supercontinent that existed between approximately 300 and 180 Ma
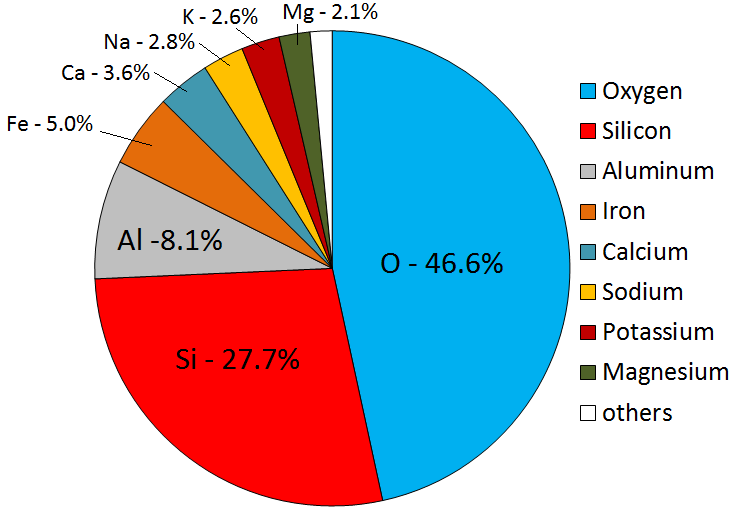
Magmas can vary widely in composition, but in general they are made up of only eight elements; in order of importance: oxygen, silicon, aluminum, iron, calcium, sodium, magnesium, and potassium (Figure 3.2.1). Oxygen, the most abundant element in magma, comprises a little less than half the total, followed by silicon at just over one-quarter. The remaining elements make up the other one-quarter. Magmas derived from crustal material are dominated by oxygen, silicon, aluminum, sodium, and potassium.
The composition of magma depends on the rock it was formed from (by melting), and the conditions of that melting. Magmas derived from the mantle have higher levels of iron, magnesium, and calcium, but they are still likely to be dominated by oxygen and silicon. All magmas have varying proportions of elements such as hydrogen, carbon, and sulphur, which are converted into gases like water vapour, carbon dioxide, and hydrogen sulphide as the magma cools.
Virtually all of the igneous rocks that we see on Earth are derived from magmas that formed from partial melting of existing rock, either in the upper mantle or the crust. Partial melting is what happens when only some parts of a rock melt; it takes place because rocks are not pure materials. Most rocks are made up of several minerals, each of which has a different melting temperature. The wax in a candle is a pure material. If you put some wax into a warm oven (50°C will do as the melting temperature of most wax is about 40°C) and leave it there for a while, it will soon start to melt. That’s complete melting, not partial melting. If instead you took a mixture of wax, plastic, aluminum, and glass and put it into the same warm oven, the wax would soon start to melt, but the plastic, aluminum, and glass would not melt (Figure 3.2.2a). That’s partial melting and the result would be solid plastic, aluminum, and glass surrounded by liquid wax (Figure 3.2.2b). If we heat the oven up to around 120°C, the plastic would melt too and mix with the liquid wax, but the aluminum and glass would remain solid (Figure 3.2.2c). Again this is partial melting. If we separated the wax/plastic “magma” from the other components and let it cool, it would eventually harden. As you can see from Figure 3.2.2d, the liquid wax and plastic have mixed, and on cooling, have formed what looks like a single solid substance. It is most likely that this is a very fine-grained mixture of solid wax and solid plastic, but it could also be some other substance that has formed from the combination of the two.

In this example, we partially melted some pretend rock to create some pretend magma. We then separated the magma from the source and allowed it to cool to make a new pretend rock with a composition quite different from the original material (it lacks glass and aluminum).
Of course partial melting in the real world isn’t exactly the same as in our pretend-rock example. The main differences are that rocks are much more complex than the four-component system we used, and the mineral components of most rocks have more similar melting temperatures, so two or more minerals are likely to melt at the same time to varying degrees. Another important difference is that when rocks melt, the process takes thousands to millions of years, not the 90 minutes it took in the pretend-rock example.
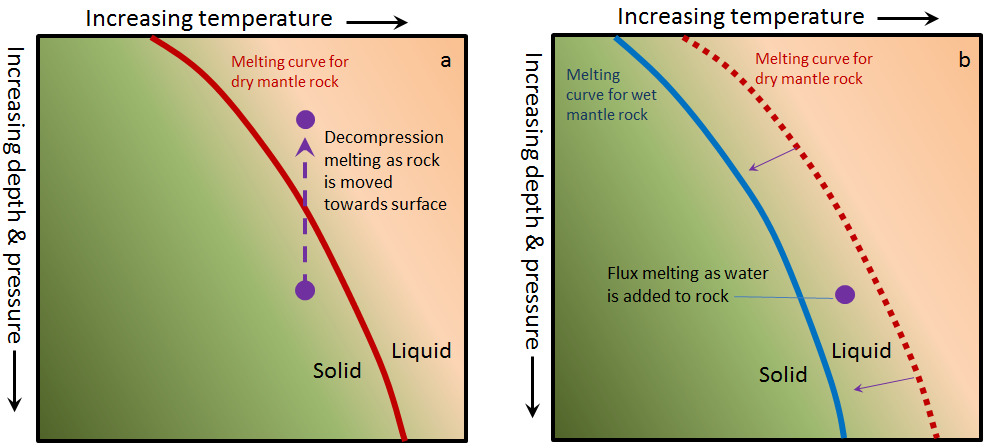
Contrary to what one might expect, and contrary to what we did to make our pretend rock, most partial melting of real rock does not involve heating the rock up. The two main mechanisms through which rocks melt are decompression melting and flux melting. Decompression melting takes place within Earth when a body of rock is held at approximately the same temperature but the pressure is reduced. This happens because the rock is being moved toward the surface, either at a mantle plume (a.k.a., hot spot), or in the upwelling part of a mantle convection cell.[1] The mechanism of decompression melting is shown in Figure 3.2.3a. If a rock that is hot enough to be close to its melting point is moved toward the surface, the pressure is reduced, and the rock can pass to the liquid side of its melting curve. At this point, partial melting starts to take place. The process of flux melting is shown in Figure 3.2.3b. If a rock is close to its melting point and some water (a flux that promotes melting) is added to the rock, the melting temperature is reduced (solid line versus dotted line), and partial melting starts.
The partial melting of rock happens in a wide range of situations, most of which are related to plate tectonics. The more important of these are shown in Figure 3.2.3. At both mantle plumes and in the upward parts of convection systems, rock is being moved toward the surface, the pressure is dropping, and at some point, the rock crosses to the liquid side of its melting curve. At subduction zones, water from the wet, subducting oceanic crust is transferred into the overlying hot mantle. This provides the flux needed to lower the melting temperature. In both of these cases, only partial melting takes place—typically only about 10% of the rock melts—and it is always the most silica-rich components of the rock that melt, creating a magma that is more silica-rich than the rock from which it is derived. (By analogy, the melt from our pretend rock is richer in wax and plastic than the “rock” from which it was derived.) The magma produced, being less dense than the surrounding rock, moves up through the mantle, and eventually into the crust.
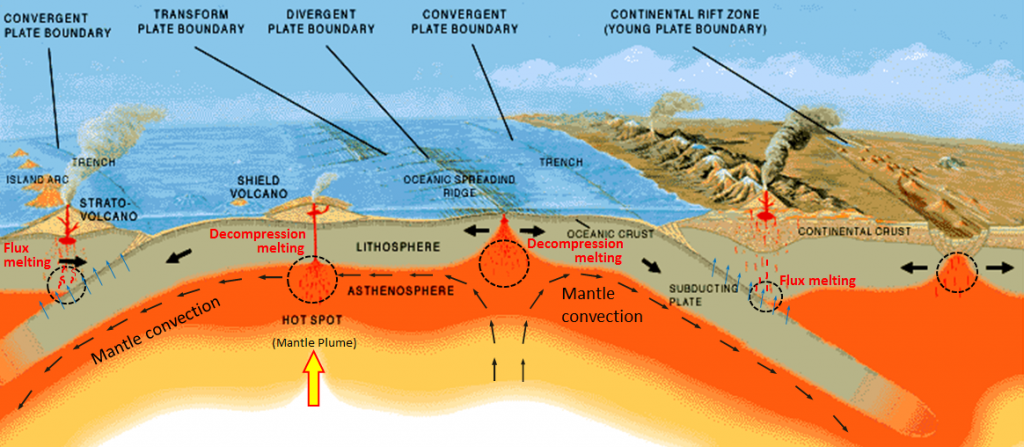
As it moves toward the surface, and especially when it moves from the mantle into the lower crust, the hot magma interacts with the surrounding rock. This typically leads to partial melting of the surrounding rock because most such magmas are hotter than the melting temperature of crustal rock. (In this case, melting is caused by an increase in temperature.) Again, the more silica-rich parts of the surrounding rock are preferentially melted, and this contributes to an increase in the silica content of the magma.
At very high temperatures (over 1300°C), most magma is entirely liquid because there is too much energy for the atoms to bond together. As the temperature drops, usually because the magma is slowly moving upward, things start to change. Silicon and oxygen combine to form silica tetrahedra, and then, as cooling continues, the tetrahedra start to link together to make chains (polymerize). These silica chains have the important effect of making the magma more viscous (less runny), and as we’ll see in Chapter 4, magma viscosity has significant implications for volcanic eruptions. As the magma continues to cool, crystals start to form.
Exercise 3.2 Making magma viscous
This is an experiment that you can do at home to help you understand the properties of magma. It will only take about 15 minutes, and all you need is half a cup of water and a few tablespoons of flour.
If you’ve ever made gravy, white sauce, or roux, you’ll know how this works.
Place about 1/2 cup (125 mL) of water in a saucepan over medium heat. Add 2 teaspoons (10 mL) of white flour (this represents silica) and stir while the mixture comes close to boiling. It should thicken like gravy because the gluten in the flour becomes polymerized into chains during this process.
Now you’re going to add more “silica” to see how this changes the viscosity of your magma. Take another 4 teaspoons (20 mL) of flour and mix it thoroughly with about 4 teaspoons (20 mL) of water in a cup and then add all of that mixture to the rest of the water and flour in the saucepan. Stir while bringing it back up to nearly boiling temperature, and then allow it to cool. This mixture should slowly become much thicker — something like porridge — because there is more gluten and more chains have been formed (see the photo).

This is analogous to magma, of course. As we’ll see below, magmas have quite variable contents of silica and therefore have widely varying viscosities (“thicknesses”) during cooling.
See Appendix 3 for Exercise 3.2 answers.
Image Descriptions
Figure 3.2.1 image description: The average elemental proportions in the Earth's crust from the largest amount to the smallest amount. Oxygen (46.6%), Silicon (27.7%), Aluminum (8.1%), Iron (5.0%), Calcium (3.6%), Sodium (2.8%), Potassium (2.6%), Magnesium (2.1%), Others (1.5%). [Return to Figure 3.2.1]
Figure 3.2.3a image description: Dry mantle rock is predominately solid. However, its melting point is dependent on the temperature and pressure the rock is under. The higher the pressure (meaning the farther the rock is from the Earth's surface), the more likely dry mantle rock is going to be solid. Dry mantle rock under extreme pressure requires a much higher temperature to melt than dry mantle rock under less pressure. As pressure drops (meaning as the rock rises towards the Earth's surface), the required temperature to melt the mantle rock drops as well.
Figure 3.2.3b image description: In comparison to dry mantle rock, wet mantle rock under the same amount of pressure (at the same distance from the earth's surface) requires a lower temperature to melt. When liquid is added to dry mantel rock at a pressure and temperature point in which wet mantle rock would be melted, flux melting occurs. [Return to Figure 3.2.3]
Media Attributions
- Figure 3.2.1, 3.2.2, 3.2.3, 3.2.5: © Steven Earle. CC BY.
- Figure 3.2.4: "Cross section" by José F. Vigil from This Dynamic Planet -- a wall map produced jointly by the U.S. Geological Survey, the Smithsonian Institution, and the U.S. Naval Research Laboratory. Adapted by Steven Earle. Public domain.
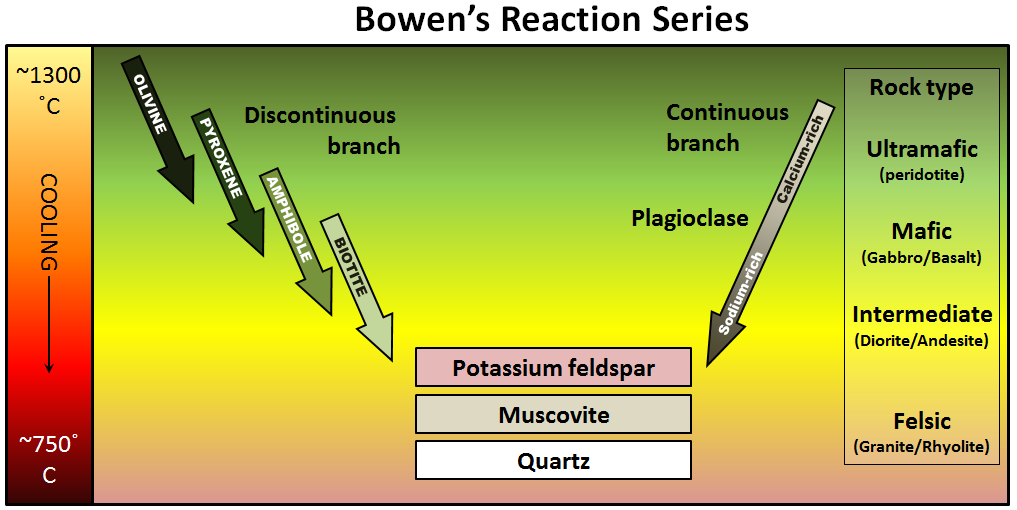
The minerals that make up igneous rocks crystallize at a range of different temperatures. This explains why a cooling magma can have some crystals within it and yet remain predominantly liquid. The sequence in which minerals crystallize from a magma is known as the Bowen reaction series (Figure 3.3.1 and Figure 3.3.3).
Of the common silicate minerals, olivine normally crystallizes first, at between 1200° and 1300°C. As the temperature drops, and assuming that some silica remains in the magma, the olivine crystals will react (combine) with some of the silica in the magma to form pyroxene. As long as there is silica remaining and the rate of cooling is slow, this process continues down the discontinuous branch: olivine to pyroxene, pyroxene to amphibole, and (under the right conditions) amphibole to biotite.
At about the point where pyroxene begins to crystallize, plagioclase feldspar also begins to crystallize. At that temperature, the plagioclase is calcium-rich (anorthite) (see Figure 2.6.1). As the temperature drops, and providing that there is sodium left in the magma, the plagioclase that forms is a more sodium-rich variety.

In some cases, individual plagioclase crystals can be zoned from calcium-rich in the centre to more sodium-rich around the outside. This occurs when calcium-rich early-forming plagioclase crystals become coated with progressively more sodium-rich plagioclase as the magma cools. Figure 3.3.2 shows a zoned plagioclase under a microscope.
Finally, if the magma is quite silica-rich to begin with, there will still be some left at around 750° to 800°C, and from this last magma, potassium feldspar, quartz, and maybe muscovite mica will form.
Who was Bowen, and what is a reaction series?

Norman Levi Bowen, born in Kingston Ontario, studied geology at Queen’s University and then at MIT in Boston. In 1912, Norman Levi Bowenhe joined the Carnegie Institution in Washington, D.C., where he carried out groundbreaking experimental research into the processes of cooling magmas. Working mostly with basaltic magmas, he determined the order of crystallization of minerals as the temperature drops. The method, in brief, was to melt the rock to a magma in a specially-made kiln, allow it to cool slowly to a specific temperature (allowing some minerals to form), and then quench it (cool it quickly) so that no new minerals form (only glass). The results were studied under the microscope and by chemical analysis. This was done over and over, each time allowing the magma to cool to a lower temperature before quenching.
The Bowen reaction series is one of the results of his work, and even a century later, it is an important basis for our understanding of igneous rocks. The word reaction is critical. In the discontinuous branch, olivine is typically the first mineral to form (at just below 1300°C). As the temperature continues to drop, olivine becomes unstable while pyroxene becomes stable. The early-forming olivine crystals react with silica in the remaining liquid magma and are converted into pyroxene, something like this:
Mg2SiO4 + SiO2 (olivine) becomes 2MgSiO3 (proxene)
This continues down the chain, as long as there is still silica left in the liquid.
The composition of the original magma is critical to magma crystallization because it determines how far the reaction process can continue before all of the silica is used up. The compositions of typical mafic, intermediate, and felsic magmas are shown in Figure 3.3.4. Note that, unlike Figure 3.2.1, these compositions are expressed in terms of “oxides” (e.g., Al2O3 rather than just Al). There are two reasons for this: one is that in the early analytical procedures, the results were always expressed that way, and the other is that all of these elements combine readily with oxygen to form oxides.
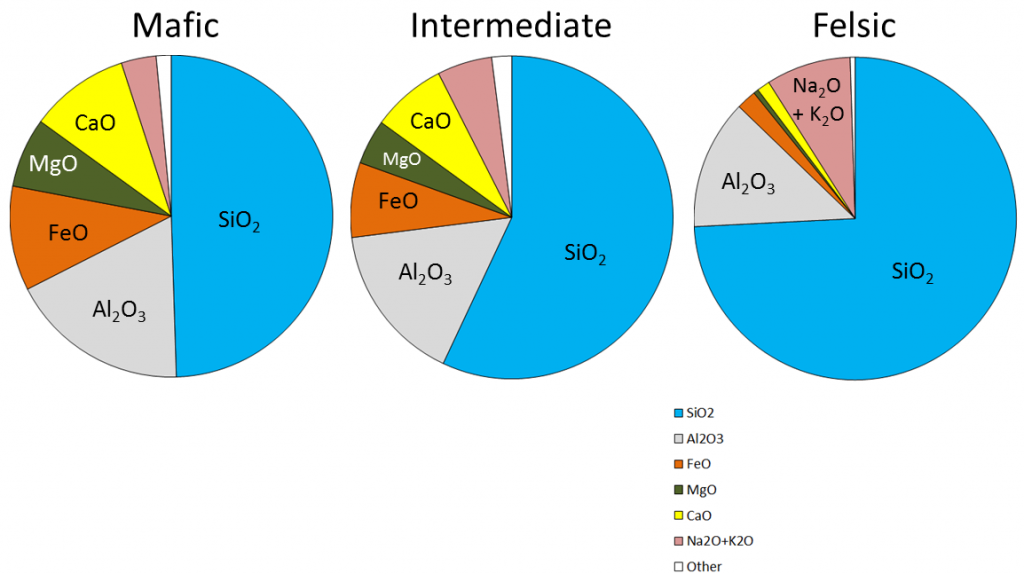
Mafic magmas have 45% to 55% SiO2, about 25% total of FeO and MgO plus CaO, and about 5% Na2O + K2O. Felsic magmas, on the other hand, have much more SiO2 (65% to 75%) and Na2O + K2O (around 10%) and much less FeO and MgO plus CaO (about 5%).
Exercise 3.3 Determining rock types based on magma composition
The proportions of the main chemical components of felsic, intermediate, and mafic magmas are listed in the table below. (The values are similar to those shown in Figure 3.3.4.)
| [Skip Table] | |||
| Oxide | Felsic Magma | Intermediate Magma | Mafic Magma |
|---|---|---|---|
| SiO2 | 65% to 75% | 55% to 65% | 45% to 55% |
| Al2O3 | 12% to 16% | 14% to 18% | 14% to 18% |
| FeO | 2% to 4% | 4% to 8% | 8% to 12% |
| CaO | 1% to 4% | 4% to 7% | 7% to 11% |
| MgO | 0% to 3% | 2% to 6% | 5% to 9% |
| Na2O | 2% to 6% | 3% to 7% | 1% to 3% |
| K2O | 3% to 5% | 2% to 4% | 0.5% to 3% |
Chemical data for four rock samples are shown in the following table. Compare these with those in the table above to determine whether each of these samples is felsic, intermediate, or mafic.
| [Skip Table] | ||||||||
| Rock Sample | SiO2 | Al2O3 | FeO | CaO | MgO | Na2O | K2O | What type of magma is it? |
|---|---|---|---|---|---|---|---|---|
| Rock 1 | 55% | 17% | 5% | 6% | 3% | 4% | 3% | |
| Rock 2 | 74% | 14% | 3% | 3% | 0.5% | 5% | 4% | |
| Rock 3 | 47% | 14% | 8% | 10% | 8% | 1% | 2% | |
| Rock 4 | 65% | 14% | 4% | 5% | 4% | 3% | 3% | |
See Appendix 3 for Exercise 3.3 answers.
As a mafic magma starts to cool, some of the silica combines with iron and magnesium to make olivine. As it cools further, much of the remaining silica goes into calcium-rich plagioclase, and any silica left may be used to convert some of the olivine to pyroxene. Soon after that, all of the magma is used up and no further changes takes place. The minerals present will be olivine, pyroxene, and calcium-rich plagioclase. If the magma cools slowly underground, the product will be gabbro; if it cools quickly at the surface, the product will be basalt (Figure 3.3.5).
Felsic magmas tend to be cooler than mafic magmas when crystallization begins (because they don’t have to be as hot to remain liquid), and so they may start out crystallizing pyroxene (not olivine) and plagioclase. As cooling continues, the various reactions on the discontinuous branch will proceed because silica is abundant, the plagioclase will become increasingly sodium-rich, and eventually potassium feldspar and quartz will form. Commonly even very felsic rocks will not have biotite or muscovite because they may not have enough aluminum or enough hydrogen to make the OH complexes that are necessary for mica minerals. Typical felsic rocks are granite and rhyolite (Figure 3.3.5).
The cooling behaviour of intermediate magmas lie somewhere between those of mafic and felsic magmas. Typical mafic rocks are gabbro (intrusive) and basalt (extrusive). Typical intermediate rocks are diorite and andesite. Typical felsic rocks are granite and rhyolite (Figure 3.3.5).

A number of processes that take place within a magma chamber can affect the types of rocks produced in the end. If the magma has a low viscosity (i.e., it’s runny)—which is likely if it is mafic—the crystals that form early, such as olivine (Figure 3.3.6a), may slowly settle toward the bottom of the magma chamber (Figure 3.3.6b). The means that the overall composition of the magma near the top of the magma chamber will become more felsic, as it is losing some iron- and magnesium-rich components. This process is known as fractional crystallization. The crystals that settle might either form an olivine-rich layer near the bottom of the magma chamber, or they might remelt because the lower part is likely to be hotter than the upper part (remember, from Chapter 1, that temperatures increase steadily with depth in Earth because of the geothermal gradient). If any melting takes place, crystal settling will make the magma at the bottom of the chamber more mafic than it was to begin with (Figure 3.3.6c).

If crystal settling does not take place, because the magma is too viscous, then the process of cooling will continue as predicted by the Bowen reaction series. In some cases, however, partially cooled but still liquid magma, with crystals in it, will either move farther up into a cooler part of the crust, or all the way to the surface during a volcanic eruption. In either of these situations, the magma that has moved toward the surface is likely to cool much faster than it did within the magma chamber, and the rest of the rock will have a finer crystalline texture. An igneous rock with large crystals embedded in a matrix of much finer crystals is indicative of a two-stage cooling process, and the texture is porphyritic (Figure 3.3.7). For the rock to be called "porphyritic" there has to be a significant difference in crystal size, where the larger crystals are at least 10 times larger than the average size of the smaller crystals.
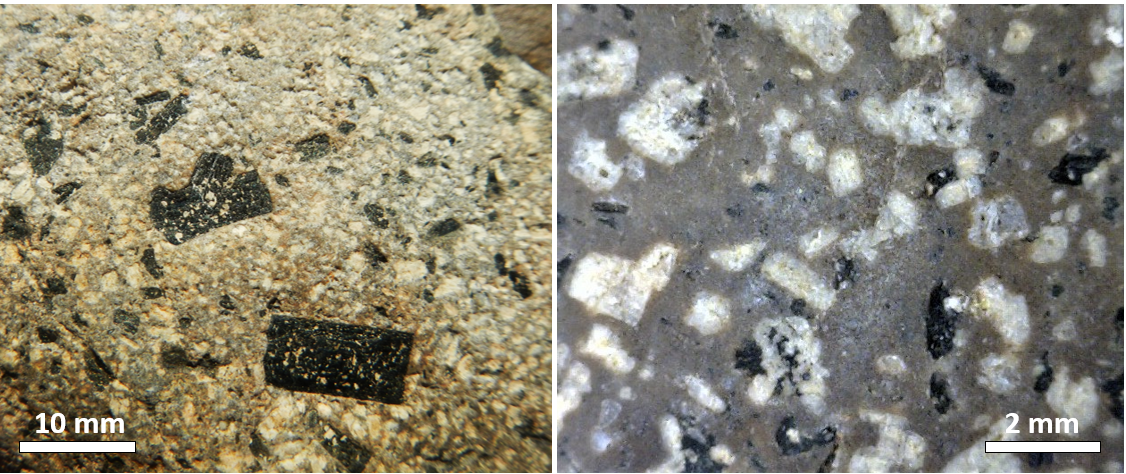
Exercise 3.4 Porphyritic minerals
As a magma cools below 1300°C, minerals start to crystallize within it. If that magma is then involved in a volcanic eruption, the rest of the liquid will cool quickly to form a porphyritic texture. The rock will have some relatively large crystals (phenocrysts) of the minerals that crystallized early, and the rest will be very fine grained or even glassy. Using Figure 3.3.8, predict what phenocrysts might be present where the magma cooled as far as line a in one case, and line b in another.

See Appendix 3 for Exercise 3.4 answers.
Media Attributions
- Figure 3.3.1, 3.3.4, 3.3.5, 3.3.6, 3.3.7, 3.3.8: © Steven Earle. CC BY.
- Figure 3.3.2: © Sandra Johnstone. CC BY.
- Figure 3.3.3: "Norman L. Bowen." Public domain.
